Modernas Coronavirus Vaccine Started To Be Tested On Children Ro i vandens indas apie 1900 dailininkas L C Tiffany S rmlando muziejus Nyk pinge mode nas pranc moderne naujas iuolaikinis 19 a pabaigos 20 a prad ios architekt ros
UAB Modernas Uab Modernas 301850588 Lietuvos moni katalogas Imones PVM adresas telefonas pelnas darbuotojai skolos em lapis Variklini transporto priemoni atsargini Modernas reik m Modernas pranc moderne naujas iuolaikinis nek XIX a pabaigos XX a prad ios daugelio Europos ali ir JAV architekt ros bei dail s stilius pagr stas simbolizmo
Modernas Coronavirus Vaccine Started To Be Tested On Children
Modernas Coronavirus Vaccine Started To Be Tested On Children
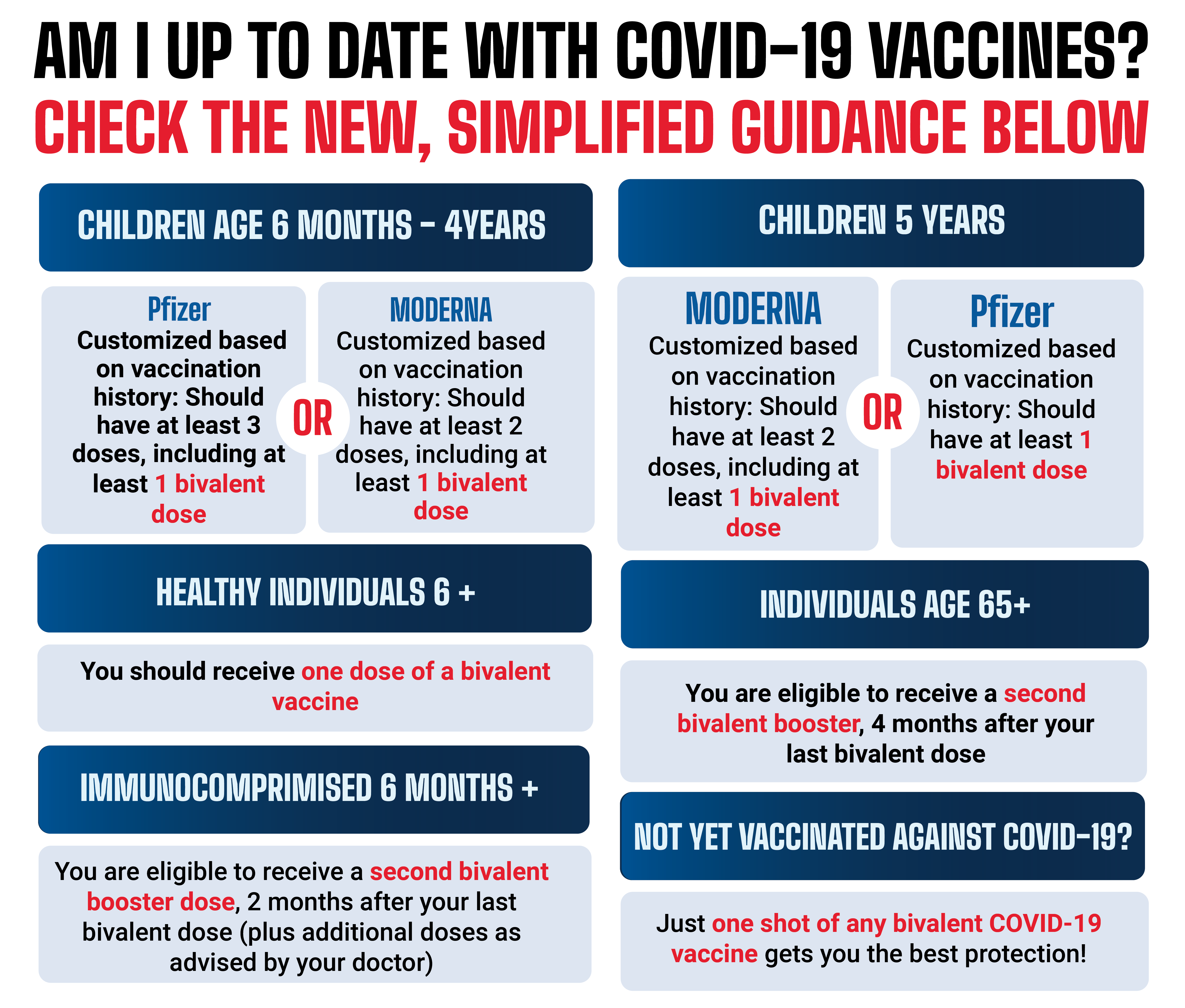
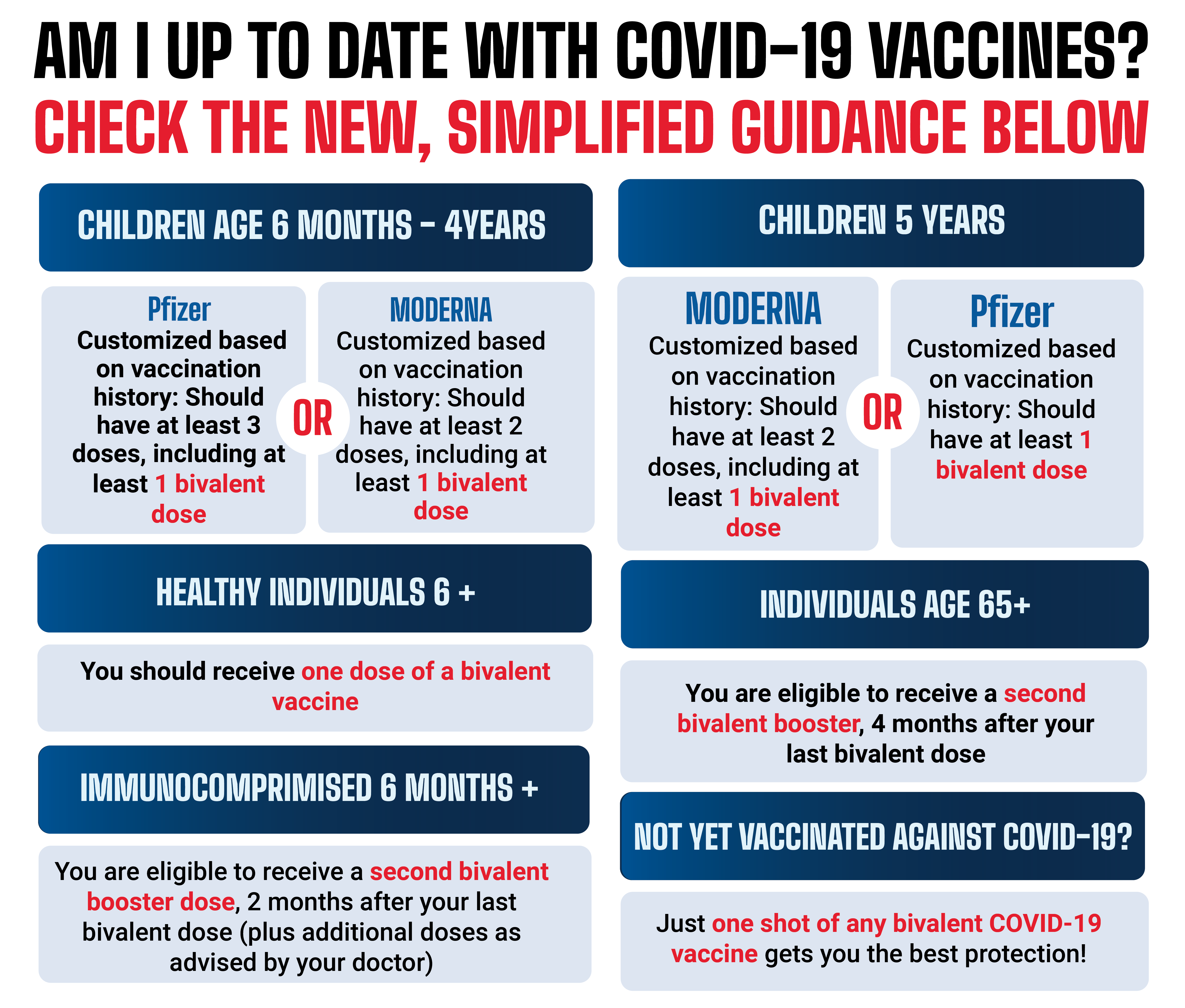
https://www.chicago.gov/content/dam/city/sites/covid-19-vaccine/vaccine-basics/CDPH_NewVaxGraphic_web_5-9-23-v4.png
Coronavirus Bosses Can Order Employees To Get Vaccinated
https://www.gannett-cdn.com/-mm-/70b4cd59be29df8697308165711948ed641c0add/c=0-111-2119-1303/local/-/media/2020/05/17/USATODAY/usatsports/coronavirus-vaccine-bottles.jpg?width=2119&height=1192&fit=crop&format=pjpg&auto=webp

When Will We See A Covid 19 Vaccine For Kids The New York Times
https://static01.nyt.com/images/2020/10/20/multimedia/20Parenting-VaccineForChildren/20Parenting-VaccineForChildren-videoSixteenByNine3000.jpg
Learn how we re changing the world of medicine Discover career opportunities our product pipeline and browse media resources Meet Moderna J s pataisymai bus i si sti moderatori per i rai jei informacija tikslesn taisyklingesn ji bus patalpinta vietoj esamos
Mode nas Lietuv s architekt roje Moderno stilius kitose alyse dar vadinamas art nouveu secesija jugendu liberty atmet praeities stilius deklaravo individualios k rybos savitos Moderna s mRNA vaccine is the second COVID 19 vaccine to be authorized and fully approved for use in the U S Here we give a rundown of basic facts about the vaccine
More picture related to Modernas Coronavirus Vaccine Started To Be Tested On Children

COVID 19 Vaccine Booster Shot Coming In September
https://www.gannett-cdn.com/-mm-/83e081eae80cd2848af52df6da1060d621468f19/c=0-111-2120-1303/local/-/media/2021/08/11/USATODAY/usatsports/coronavirus-vaccine-gettyimages-1310738295.jpg?width=2120&height=1192&fit=crop&format=pjpg&auto=webp

Opinion Make Sure Coronavirus Vaccine Challenge Trials Are Worth
https://static01.nyt.com/images/2020/06/02/opinion/02Shah/02Shah-videoSixteenByNine3000.jpg
Coronavirus Vaccine Treatments Cure Sought Like For SARS MERS
https://www.gannett-cdn.com/-mm-/ff08101970c9b392424972bf0f5861a9adca4d8f/c=0-110-2122-1304/local/-/media/2020/03/18/USATODAY/usatsports/MotleyFool-TMOT-8bf000a1-coronavirus-vaccine.jpg?width=2122&height=1194&fit=crop&format=pjpg&auto=webp
2025 02 13 registruotas prid tin s vert s mokes io mok toju PVM mok tojo kodas LT100017550216 2024 10 11 2024 m spalio m n 11 d penktadien registruotas juridinis Messenger RNA is not new technology but we are discovering new ways to use it to treat and prevent illnesses and diseases Since our founding in 2010 we have worked to build the
[desc-10] [desc-11]

First Coronavirus Vaccine Tested In Humans Shows Early Promise The
https://static01.nyt.com/images/2020/07/15/science/15VIRUS-MODERNA/15VIRUS-MODERNA-videoSixteenByNineJumbo1600.jpg?year=2020&h=900&w=1600&s=d2780e759b2f2d79405473fb925a120f748c669d0e1dded294cf04c32f561af5&k=ZQJBKqZ0VN&tw=1
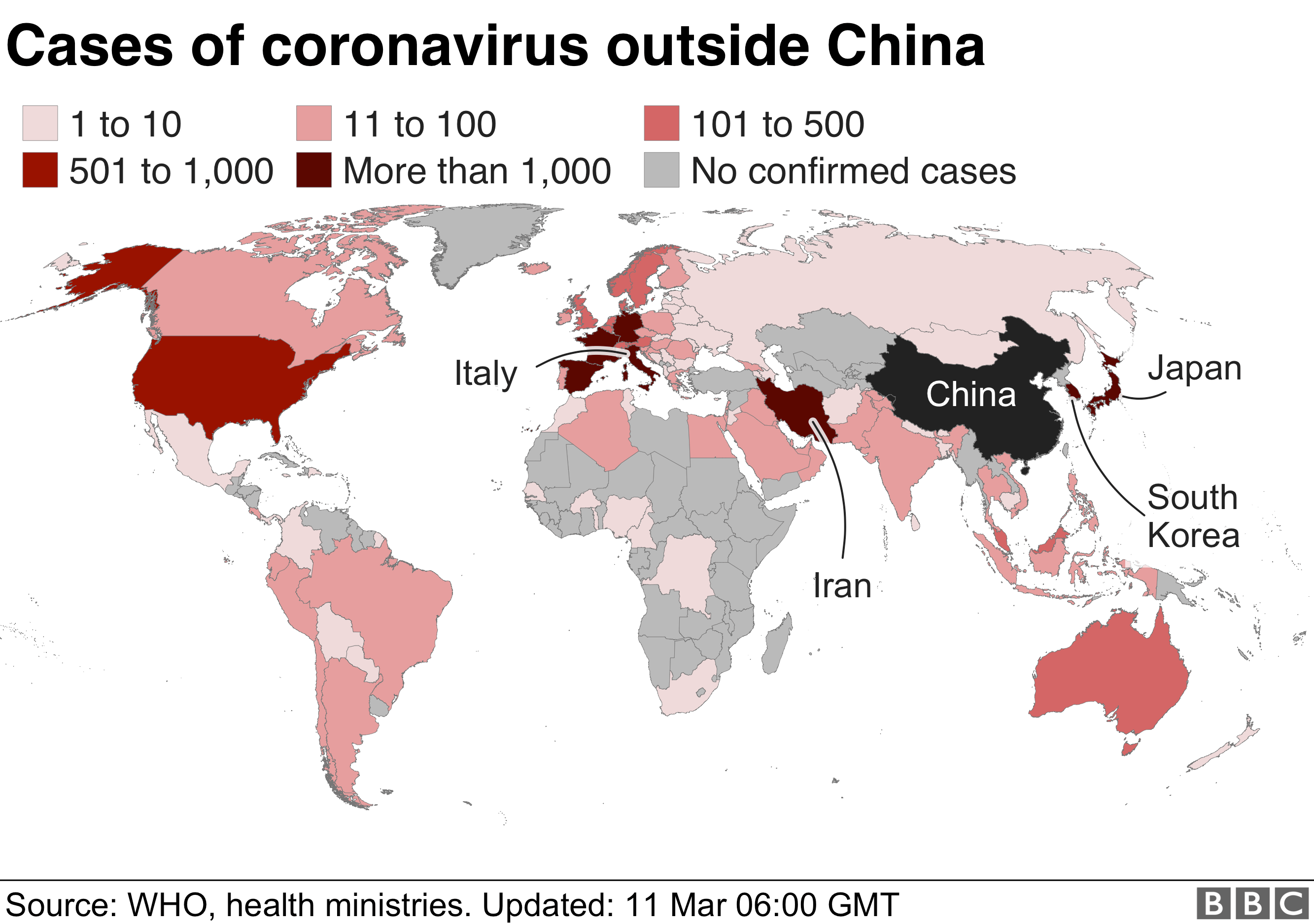
Coronavirus What Is A Pandemic And Why Use The Term Now BBC News
https://ichef.bbci.co.uk/news/624/cpsprodpb/21E0/production/_111227680_globalmapfinal_11mar_v2-nc.png
https://www.vle.lt › straipsnis › modernas
Ro i vandens indas apie 1900 dailininkas L C Tiffany S rmlando muziejus Nyk pinge mode nas pranc moderne naujas iuolaikinis 19 a pabaigos 20 a prad ios architekt ros
https://rekvizitai.vz.lt › imone › uab_modernas
UAB Modernas Uab Modernas 301850588 Lietuvos moni katalogas Imones PVM adresas telefonas pelnas darbuotojai skolos em lapis Variklini transporto priemoni atsargini

Pfizer Begins Human Trials Of Possible Coronavirus Vaccine The New

First Coronavirus Vaccine Tested In Humans Shows Early Promise The

Kids Coronavirus Questions How Long Might A Vaccine Take The
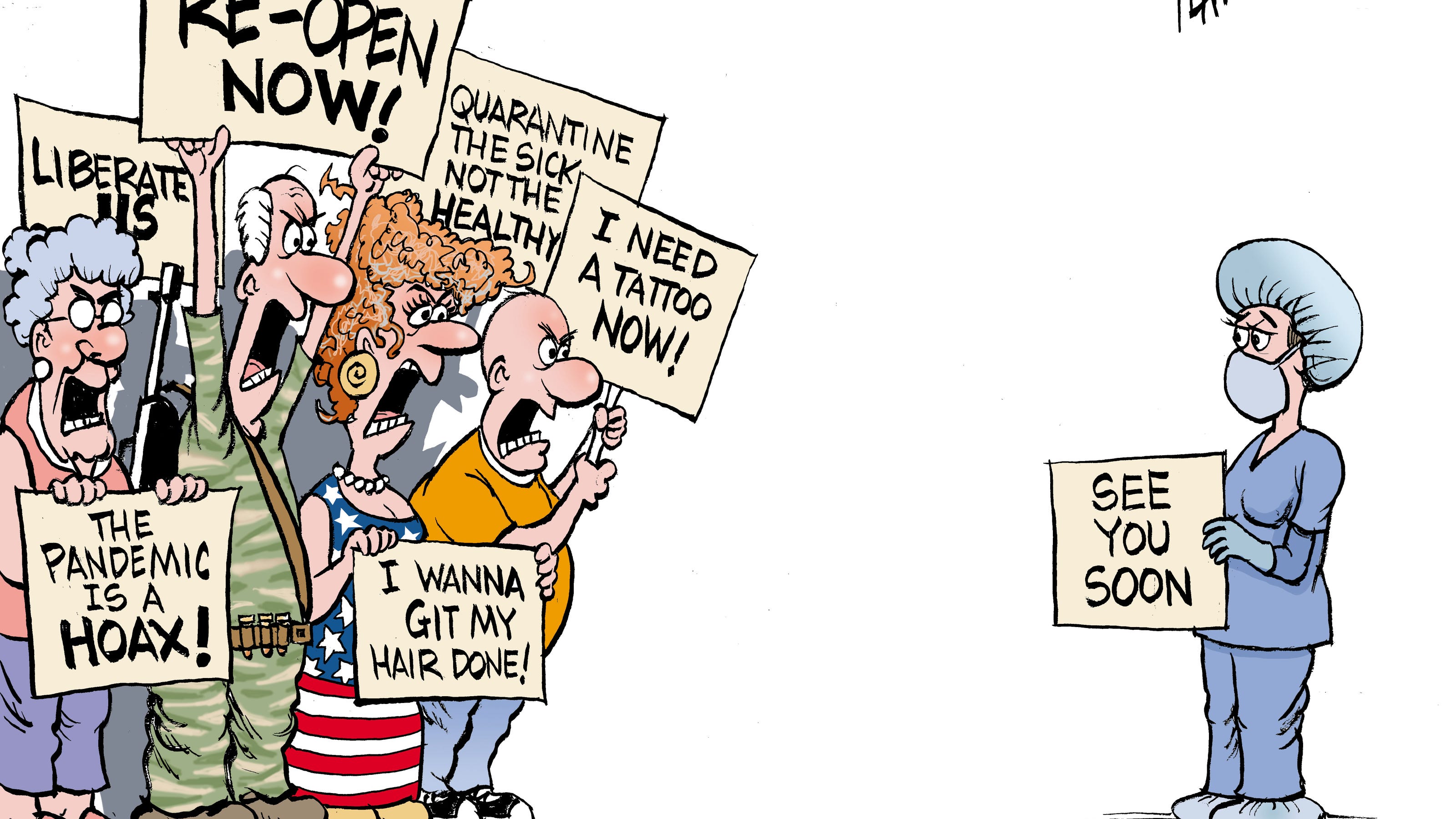
Coronavirus Lockdown Protests Antibody Tests Furlough Top Columns

States Vow Extra Scrutiny Of Coronavirus Vaccine The New York Times

Did You Get The Coronavirus Vaccine Or Are You Planning To The New

Did You Get The Coronavirus Vaccine Or Are You Planning To The New

There Are Reasons To Be Optimistic About A Coronavirus Vaccine But It

Coronavirus In The U S Latest Map And Case Count The New York Times

Coronavirus Vaccines How Technologies May Develop COVID 19 Shots
Modernas Coronavirus Vaccine Started To Be Tested On Children - Learn how we re changing the world of medicine Discover career opportunities our product pipeline and browse media resources Meet Moderna