11 Times 12 50 PAI coverage is based on the totality of information available to FDA about the site which can include information from previous inspections of the establishment
What is a Pre Approval Inspection The FDA conducts PAIs to assure that a manufacturing site named in a drug application can manufacture the product and that the data Current minimum standards for methods to be used in and facilities or controls to be used for the manufacture processing packing or holding of a drug to assure that it meets its required
11 Times 12 50
11 Times 12 50
https://timestablesworksheets.com/wp-content/uploads/2020/10/times-table-exercise-basic-times-tables-worksheets-for-multiplication-table-study-sheet-1.jpg
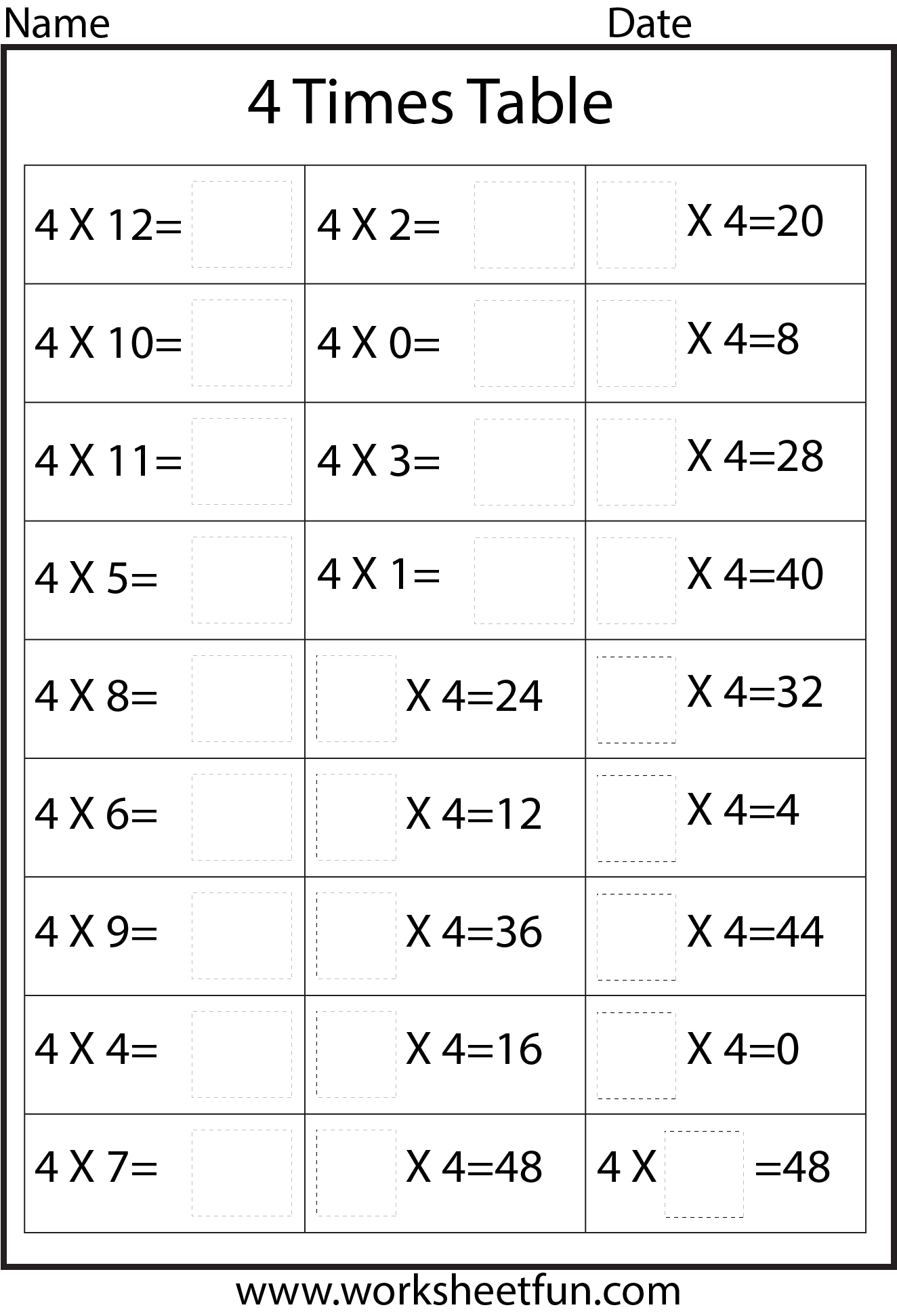
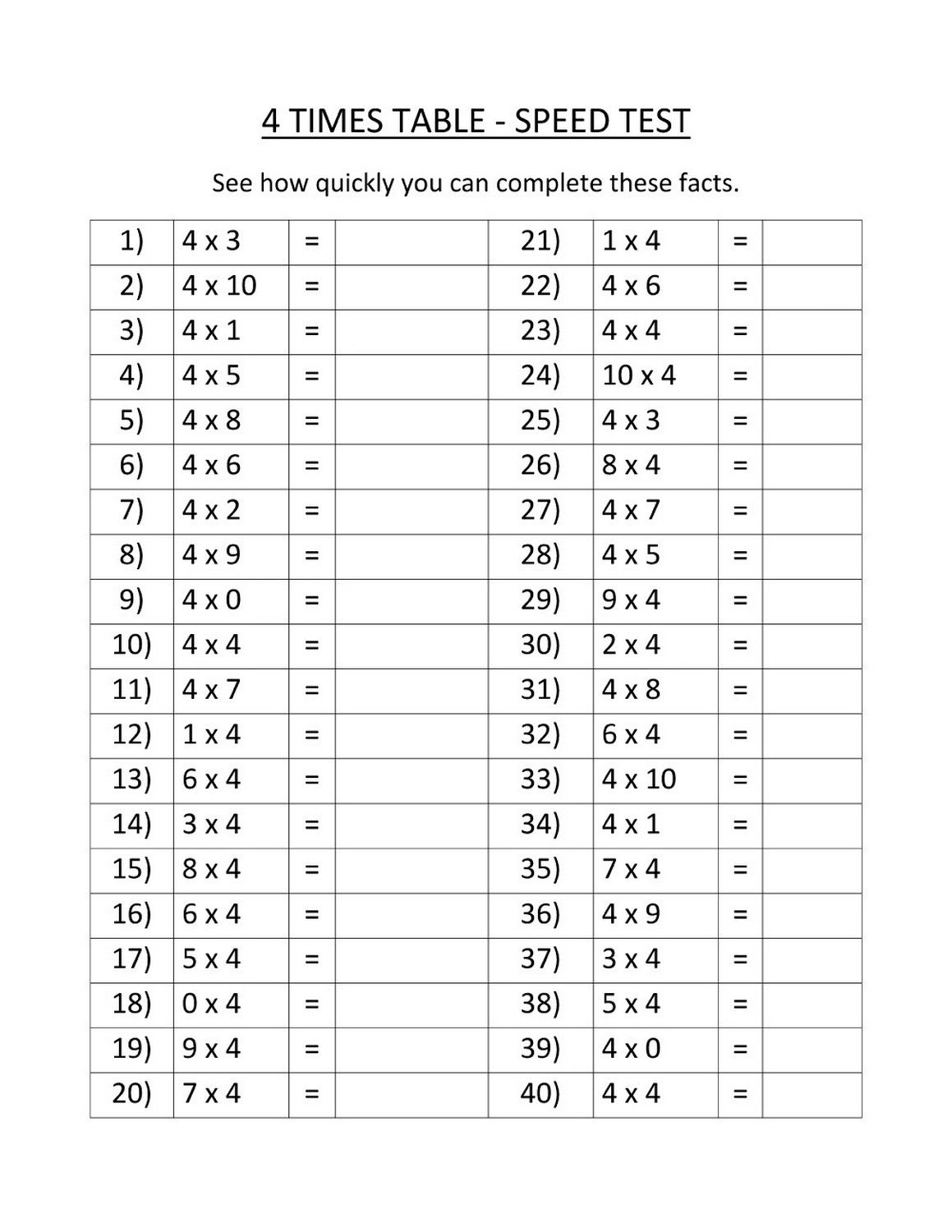
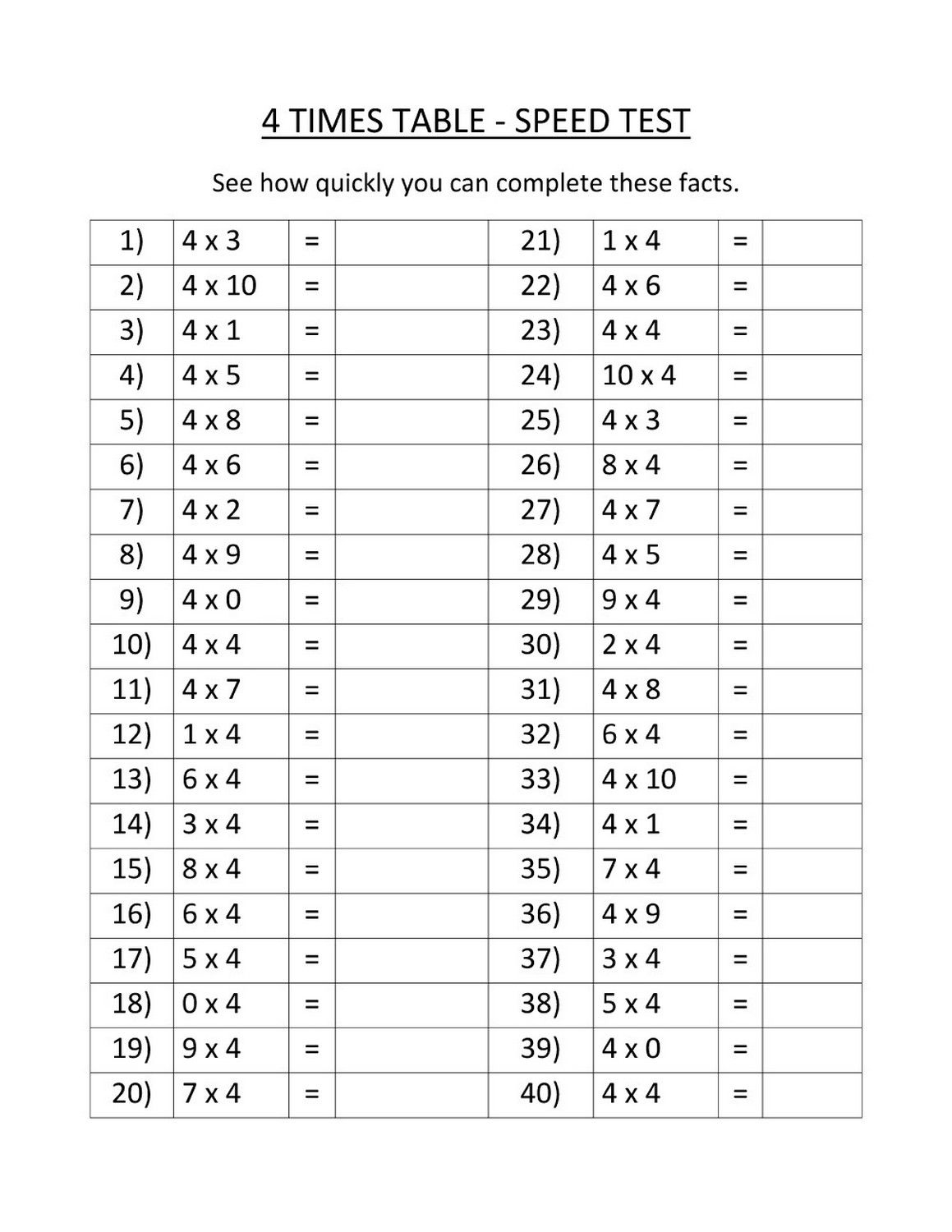
Time Table 4 Worksheet
https://timestablesworksheets.com/wp-content/uploads/2020/10/multiplication-basic-facts-2-3-4-5-6-7-8-9-12-in-4-times-table-answers-1.png
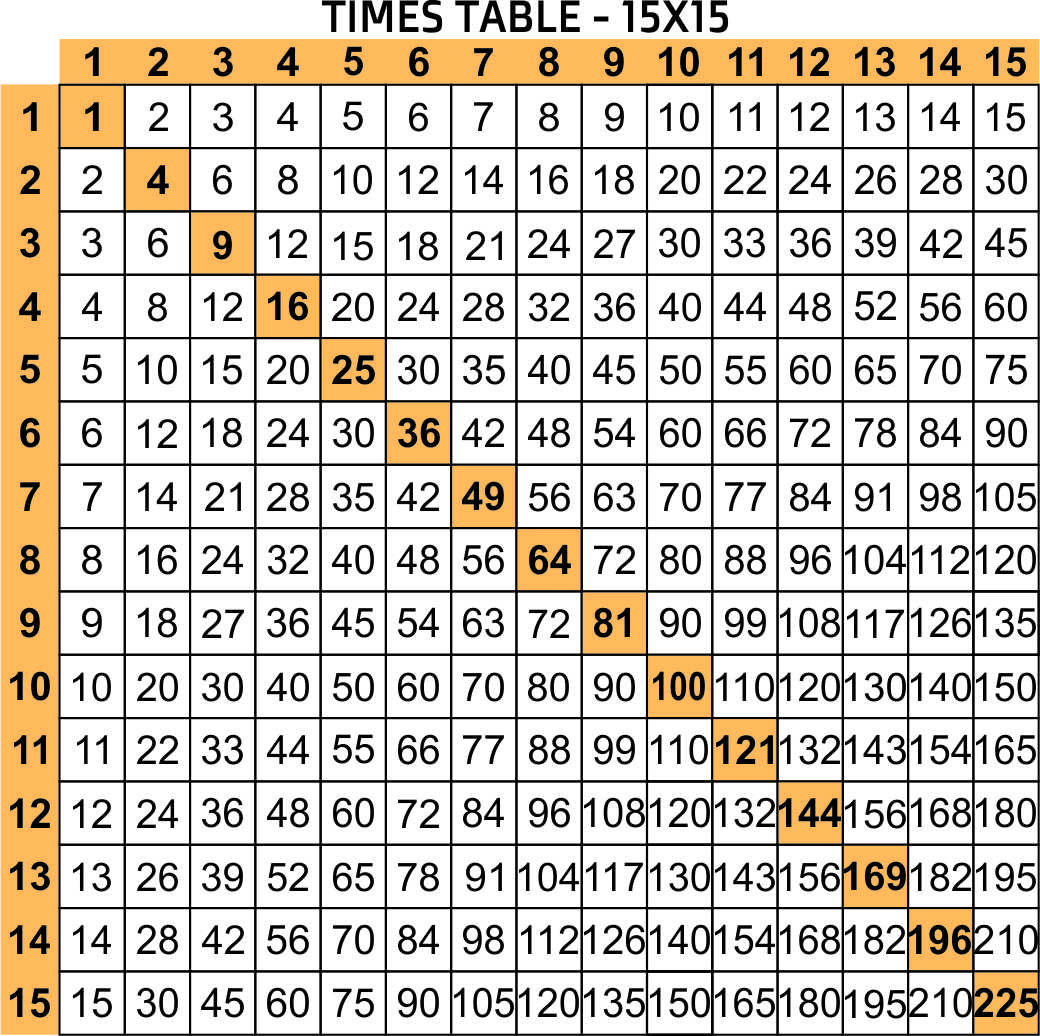
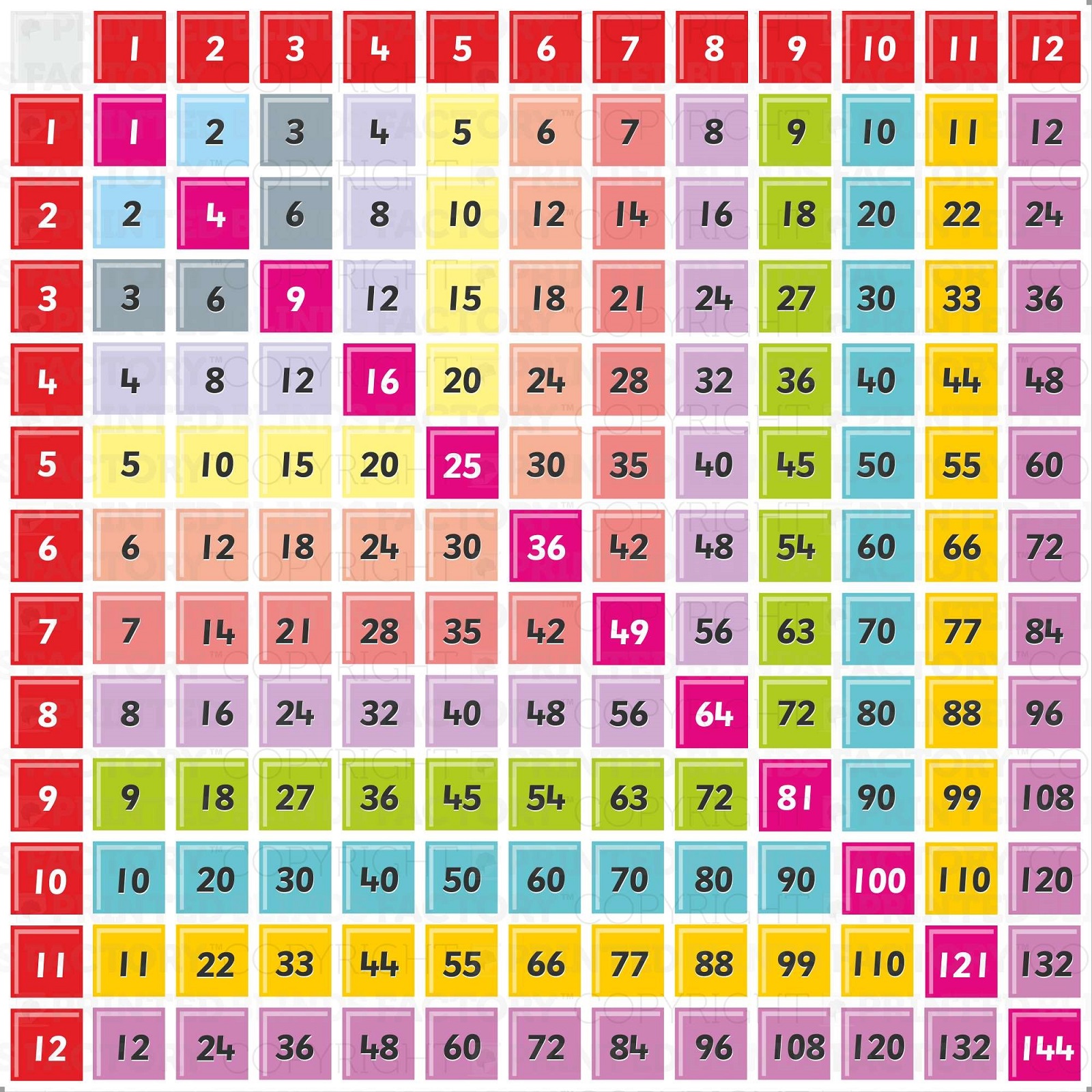
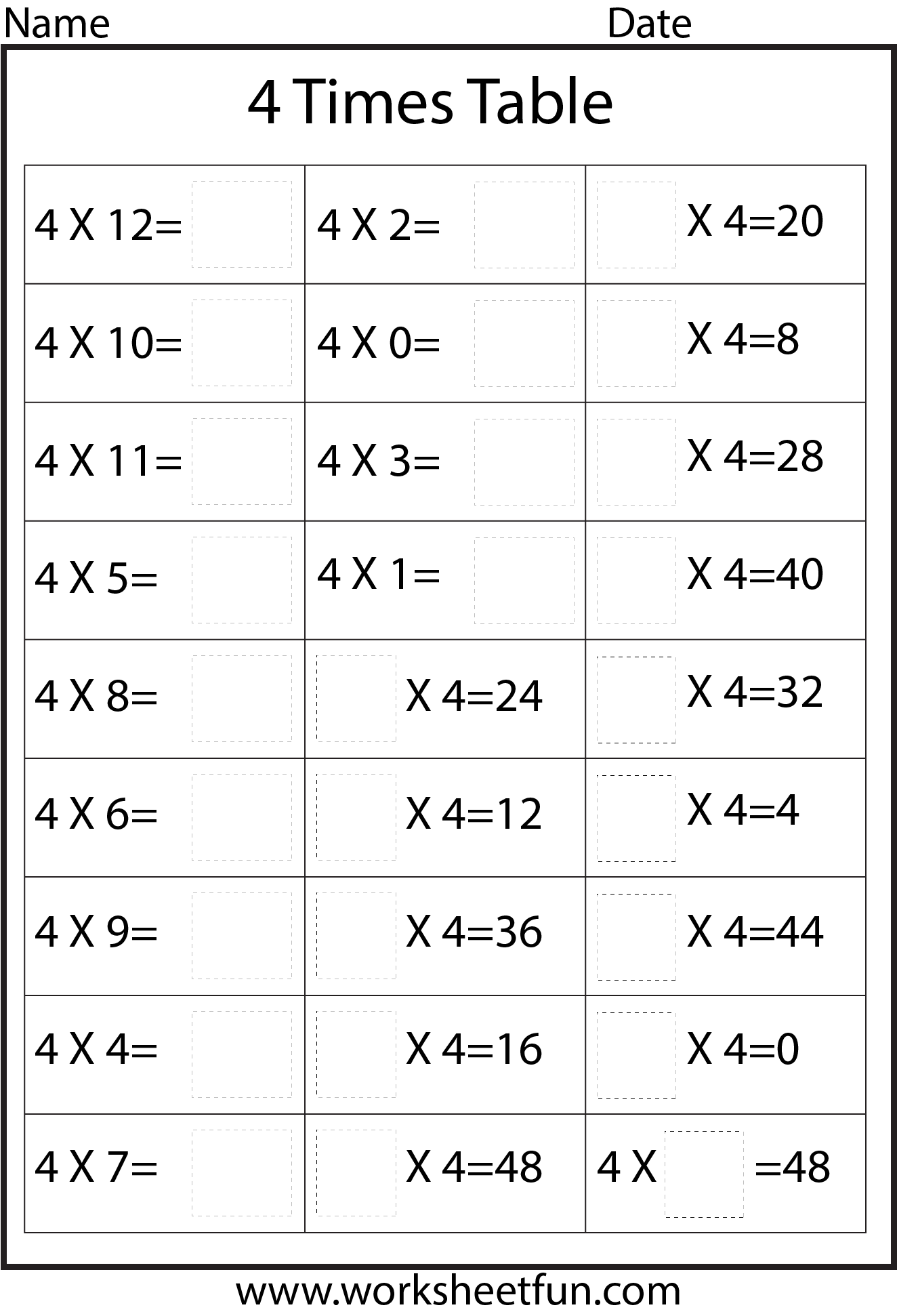
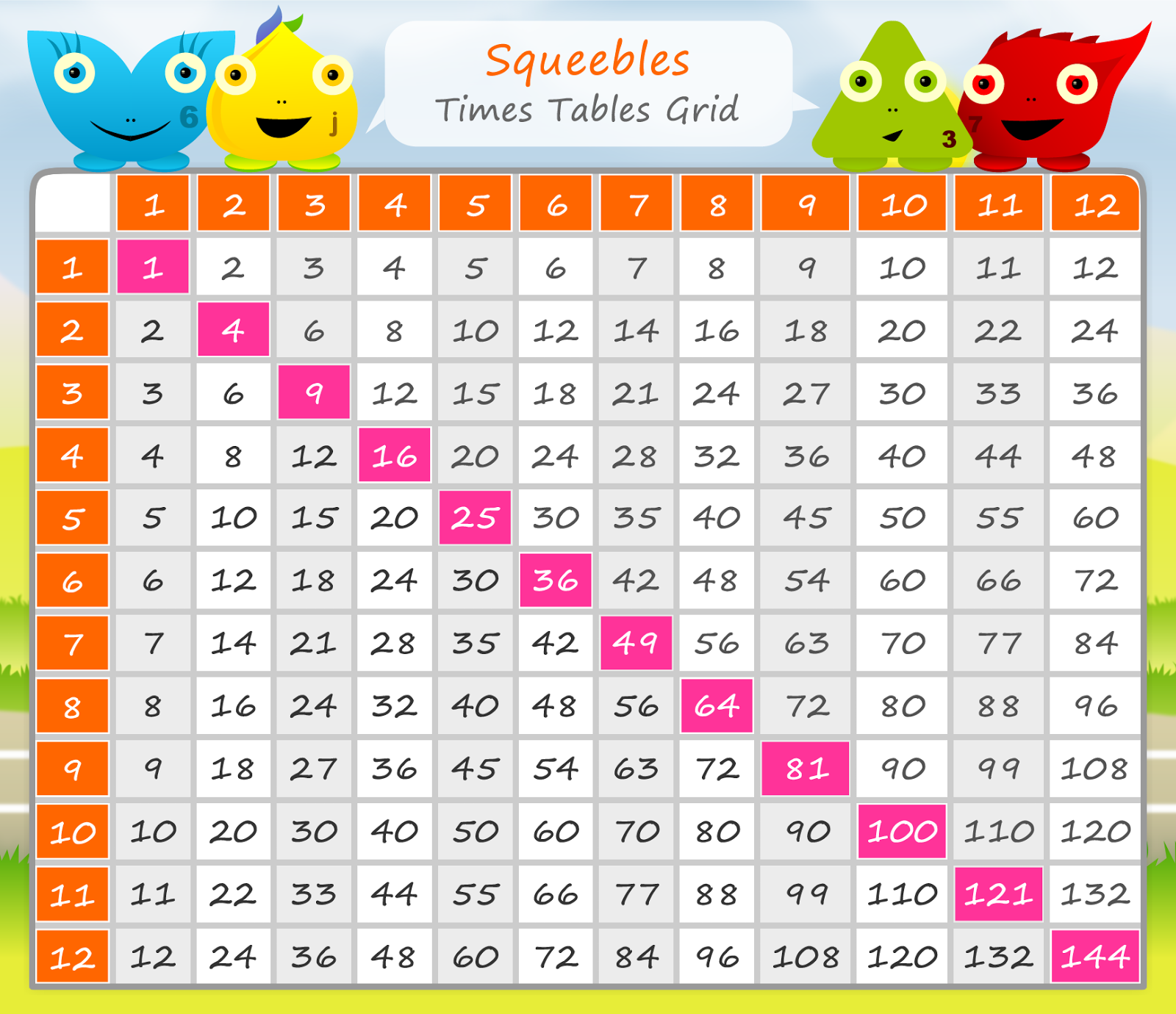
Chart Of Times Tables
https://printablee.com/postpic/2012/05/multiplication-times-table-chart-15_219474.jpg
A pre approval inspection PAI is performed to contribute to FDA s assurance that a manufacturing establishment named in a drug application is capable of manufacturing a drug US FDA S PRE APPLICATION INSPECTIONS WHAT IS PRE APPROVAL INSPECTION A pre approval inspection PAI is performed to contribute to FDA s assurance that a manufacturing
What is a Pre Approval Inspection PAI A pre approval inspection PAI is performed to provide the Food and Drug Administration FDA assurances that a In the realm of quality and compliance within pharmaceuticals biopharmaceuticals and combination products I ve frequently been asked about the crucial steps to pass an FDA Pre
More picture related to 11 Times 12 50
Printable Multiplication Table Charts
http://www.activityshelter.com/wp-content/uploads/2017/08/times-tables-chart-multiplication.jpg

Show Me A Picture Of A Multiplication Chart
http://www.primrosehill.camden.sch.uk/wp-content/uploads/2015/03/times-tables-grid4.png

2 5 And 10 Times Tables Worksheets
https://i.pinimg.com/originals/47/bb/6d/47bb6dd819ff484f90c3a4d339c28a58.jpg
The Pre Approval Inspection PAI is perhaps the most pivotal moment in a drug s journey to market Recent updates to the FDA s compliance program guides have modernized Preapproval and postmarket PMA QS inspections should generally be conducted using the Quality System Inspection Technique QSIT Guidance for performing an inspection is
A pre approval inspection PAI is performed to contribute to FDA s assurance that a manufacturing establishment named in a drug application is capable of manufacturing a A Pre Approval Inspection PAI is an on site evaluation conducted by the FDA to ensure that a facility listed in a drug application can produce a product that meets regulatory standards
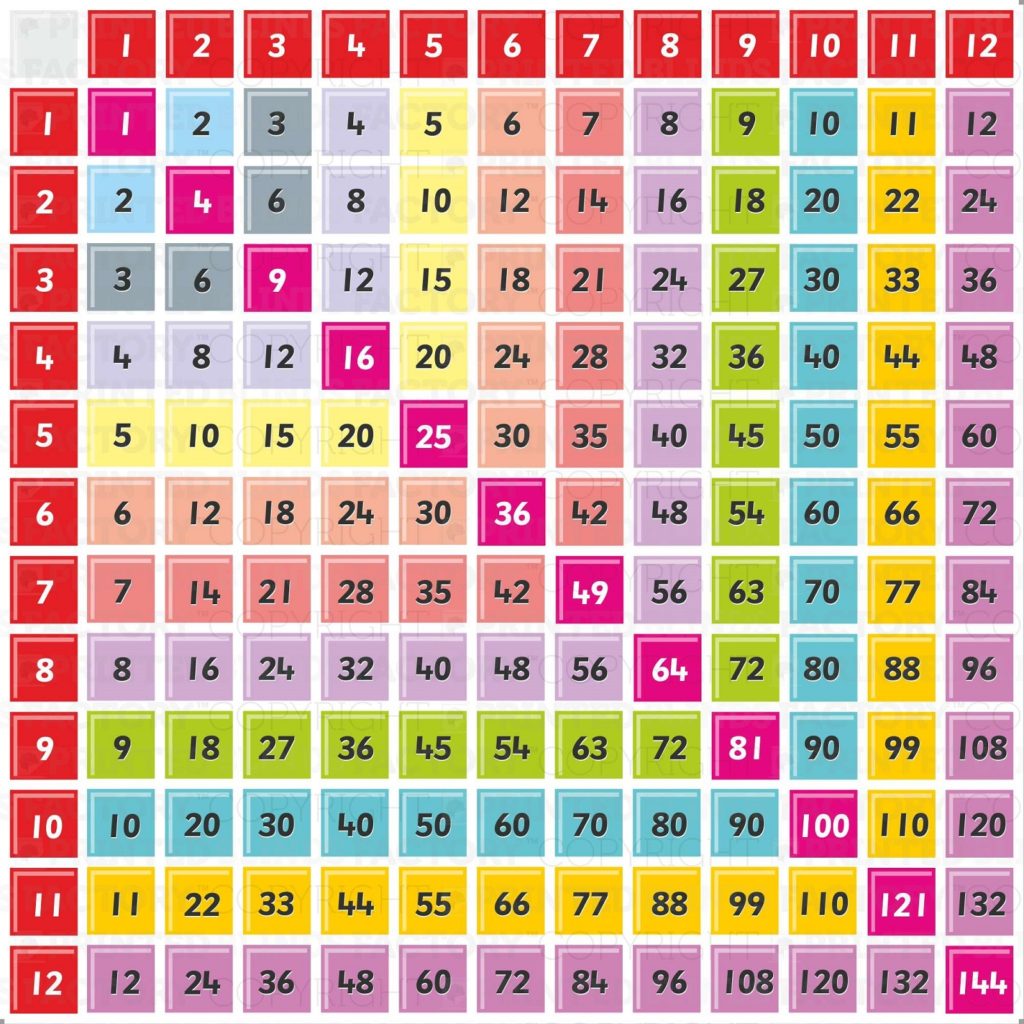
6 Times Tables Chart Up To 100
https://www.activityshelter.com/wp-content/uploads/2017/08/times-tables-chart-multiplication-1024x1024.jpg

Free Printable Full Size Times Table Chart
https://cdn.shopify.com/s/files/1/0022/9303/9202/products/Learning-Multiplication-table-chart-LAMINATED-poster-for-classroom-clear-teaching-tool-for-schools-B074XJBJYX-3.jpg?v=1546026097
https://www.fda.gov › media › download
PAI coverage is based on the totality of information available to FDA about the site which can include information from previous inspections of the establishment
https://www.thefdagroup.com › blog › pre-approval...
What is a Pre Approval Inspection The FDA conducts PAIs to assure that a manufacturing site named in a drug application can manufacture the product and that the data
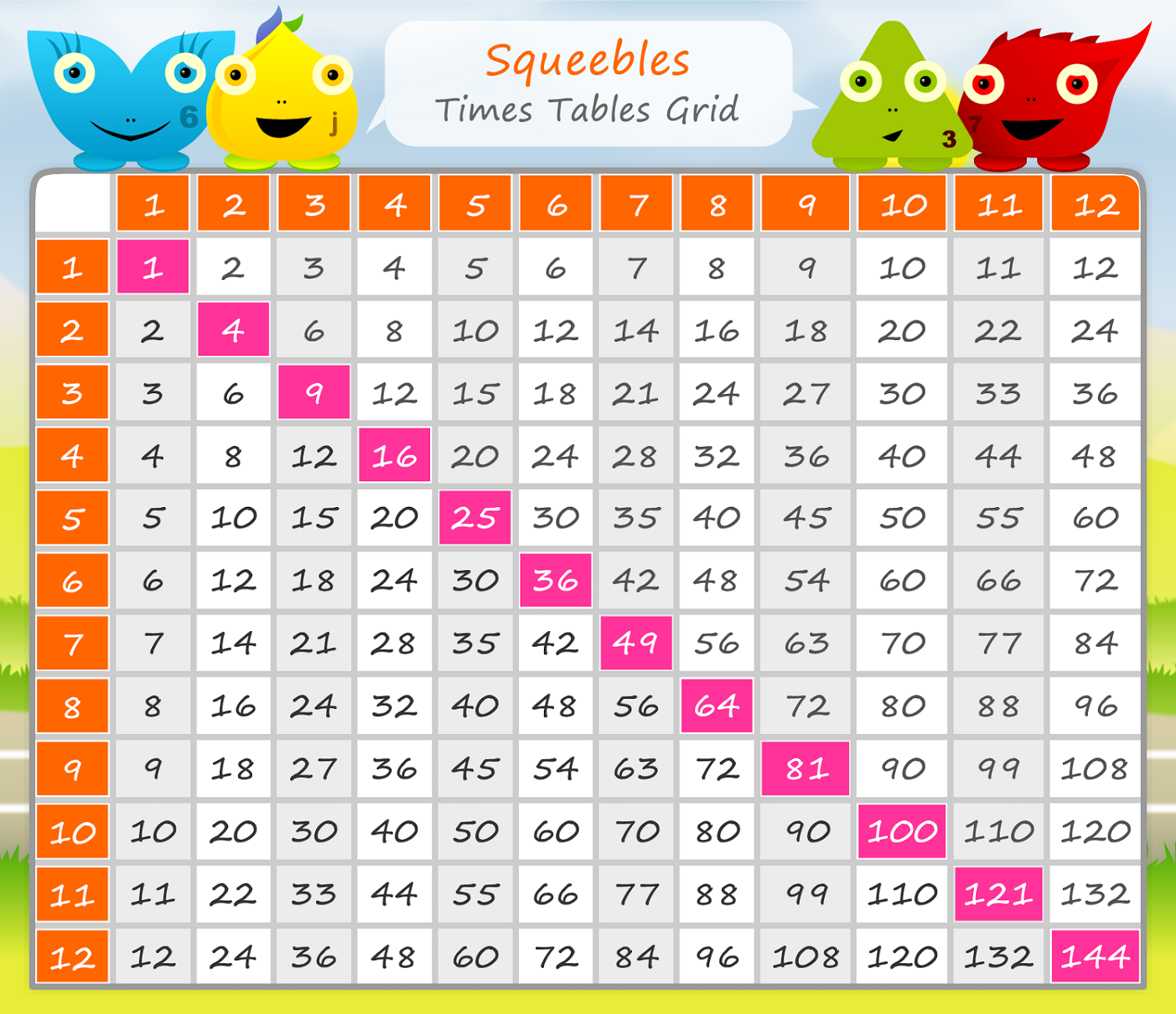
12 Times Table Up To 20 Horfact

6 Times Tables Chart Up To 100

Time Tables Practice Sheets

Times Front Page 26th Of August 2023 Tomorrow s Papers Today
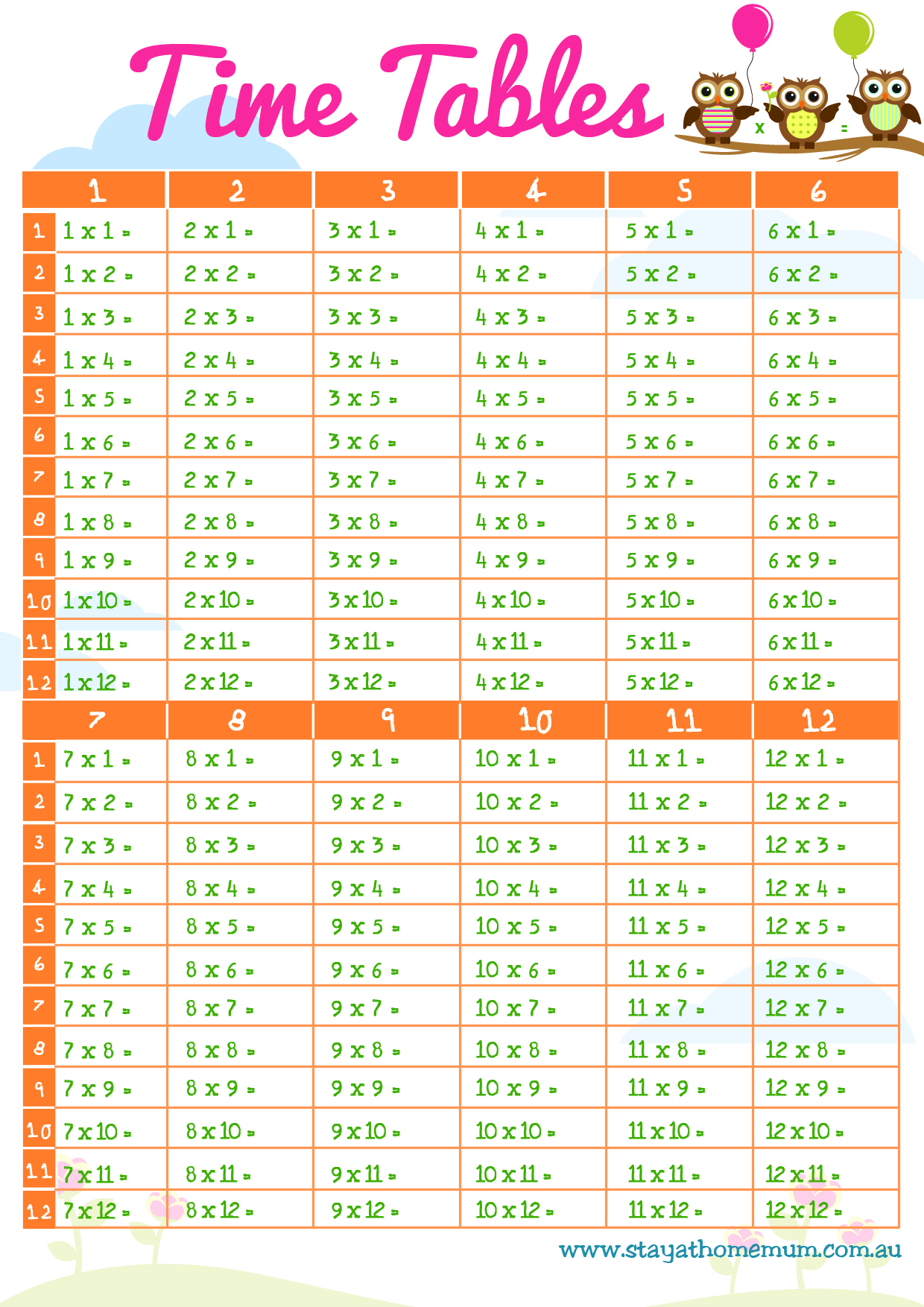
Times Table Chart Free Printable Fill In
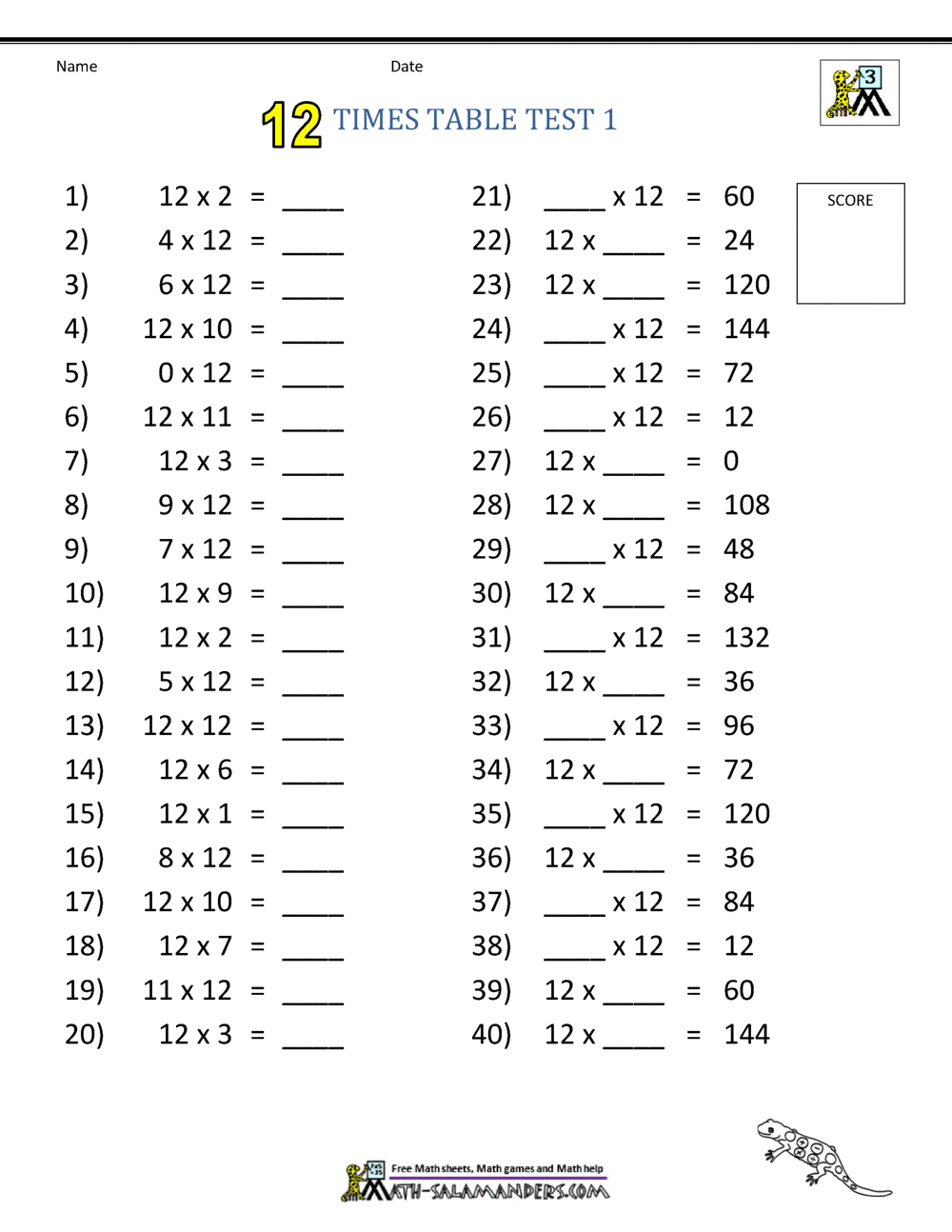
Multiplication Worksheets 12 Times Tables

Multiplication Worksheets 12 Times Tables
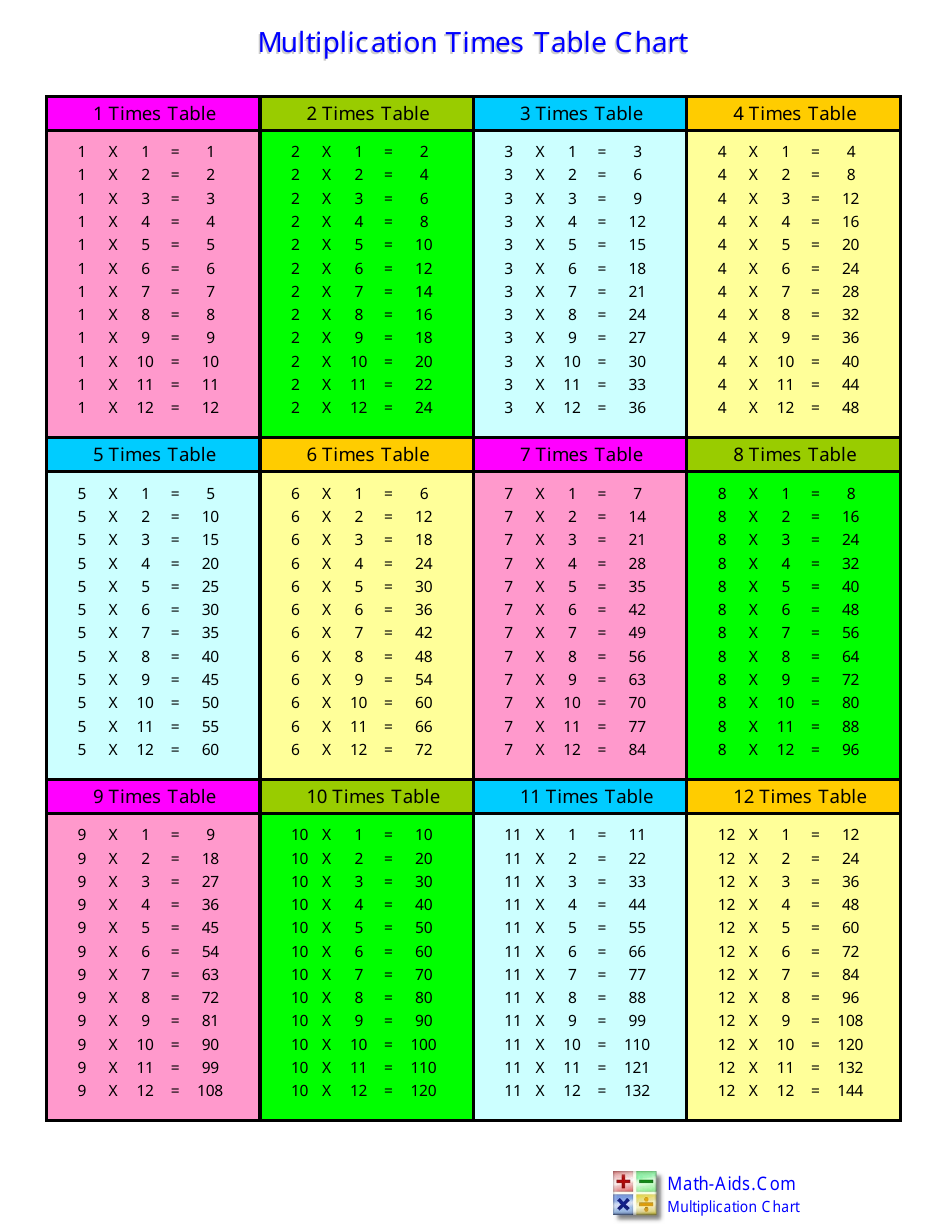
Times Tables Chart Free Printable
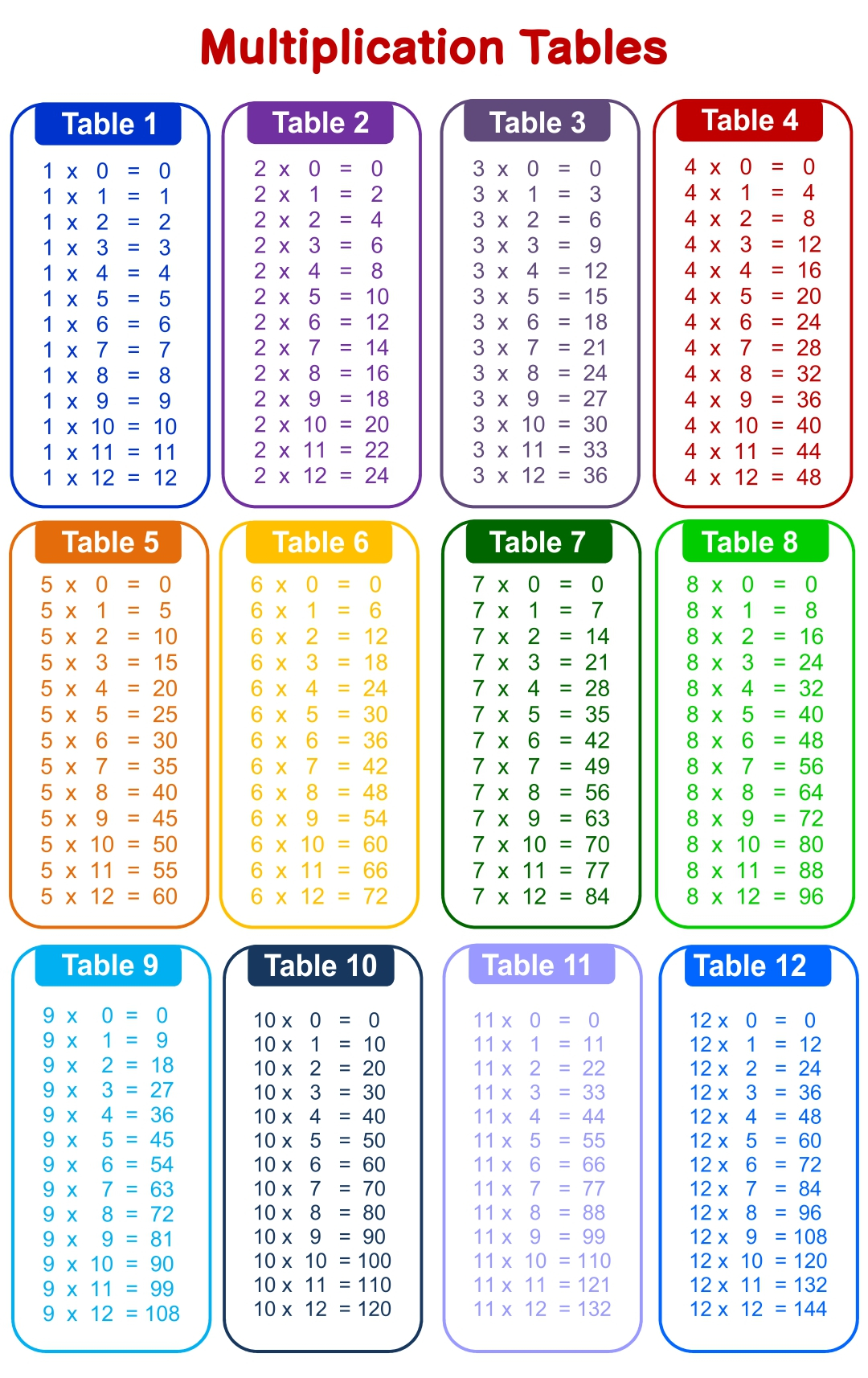
Printable Table Of Multiplication
Times Tables New Calendar Template Site
11 Times 12 50 - The FDA conducts Pre approval Inspections to assure that a manufacturing site named in a drug application can manufacture the product and that the data submitted in the