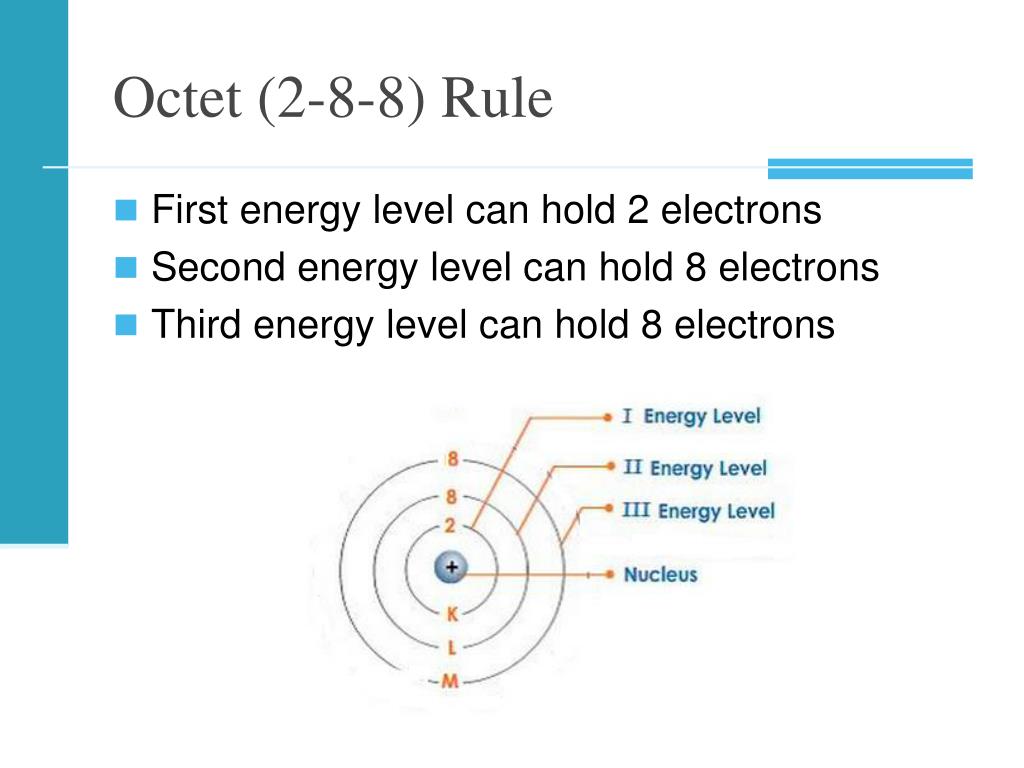
2 8 8 Rule What is the 2 8 8 rule for electron configuration The 2 8 8 rule also known as the octet rule states that atoms tend to gain lose or share electrons to achieve a stable electron configuration with 8 electrons in their outermost shell
We use the orbital energy diagram of Figure 2 8 1 2 8 1 recognizing that each orbital can hold two electrons one with spin up corresponding to ms which is arbitrarily written first and one with spin down corresponding to ms A filled orbital is indicated by in which the electron spins are said to be paired Using this rule Bohr and others determined the electron configurations which electrons were in which state or orbit of most of the elements and found that the order matched the periodic table nicely
2 8 8 Rule

2 8 8 Rule
https://image.slidesharecdn.com/bonding-110409051041-phpapp01/95/bonding-4-728.jpg?cb=1302325934

What Is The 2 8 8 18 Rule In Chemistry YouTube
https://i.ytimg.com/vi/tLY6f-5imr0/maxresdefault.jpg

The 8 Second Rule
https://www.properti.ai/wp-content/uploads/2022/06/Podcast-5.png
The correct answer is 2 i e 2 8 8 18 18 32 It is an arrangement of electrons in various shells sub shells and orbitals in an atom It is written as 2 8 8 18 18 32 It is written as nlx where n indicates the principal quantum number l indicates the azimuthal quantum number or sub shell and x is the number of electrons The 2 8 8 rule also known as the octet rule pertains to the electron configuration of elements in the periodic table It states that the outermost electron shell of an atom can hold a maximum of 8 electrons
The maximum number of electrons per shell in order of increasing shell number from 1 to 4 was said to be respectively 2 8 8 and 18 An atom will be made of the same number of electron shells as the number of period where it is found in the Periodic Table The 2 8 8 rule is a simplified model used in introductory chemistry to predict the electron configuration of atoms specifically those with atomic numbers 1 18 It describes how electrons fill the principal energy levels shells around an atom s nucleus
More picture related to 2 8 8 Rule

Bonding
https://image.slidesharecdn.com/bonding-110409051041-phpapp01/95/bonding-8-728.jpg?cb=1302325934

The 4 Rule Explained
https://learnwithdrscott.com/wp-content/uploads/2020/11/octet-rule-covalent-bonds-nonmetal-elements-Ver2.jpg
PPT Week 9 CCA Test Review PowerPoint Presentation Free Download
https://image3.slideserve.com/6989595/octet-2-8-8-rule-l.jpg
Demystifying the 2 8 8 18 Rule in Chemistry 2 8 8 18 Rule Learn about the significance of the 2 8 8 18 rule in chemistry which explains how electrons are distributed in atomic Bohr figured out the number of electrons in each shell where a shell is all the electrons with the same principal quantum number The pattern he used which you can verify with the periodic table was 2 8 8 18 18 32 32
[desc-10] [desc-11]

8 1 Part 2 Finding A Rule YouTube
https://i.ytimg.com/vi/usBFFjfVJ3Y/maxresdefault.jpg

8 8 8 RULE YouTube
https://i.ytimg.com/vi/8DJqO7K0GPI/maxres2.jpg?sqp=-oaymwEoCIAKENAF8quKqQMcGADwAQH4Ac4FgAKACooCDAgAEAEYTCBhKGUwDw==&rs=AOn4CLAVb-WabONcr9_NCkcrEwKsNGIaeQ

https://www.studypug.com › chemistry-help
What is the 2 8 8 rule for electron configuration The 2 8 8 rule also known as the octet rule states that atoms tend to gain lose or share electrons to achieve a stable electron configuration with 8 electrons in their outermost shell

https://chem.libretexts.org › Courses › Valley_City_State_University
We use the orbital energy diagram of Figure 2 8 1 2 8 1 recognizing that each orbital can hold two electrons one with spin up corresponding to ms which is arbitrarily written first and one with spin down corresponding to ms A filled orbital is indicated by in which the electron spins are said to be paired

8 8 8 Rule YouTube

8 1 Part 2 Finding A Rule YouTube

8 8 8 RULE YouTube
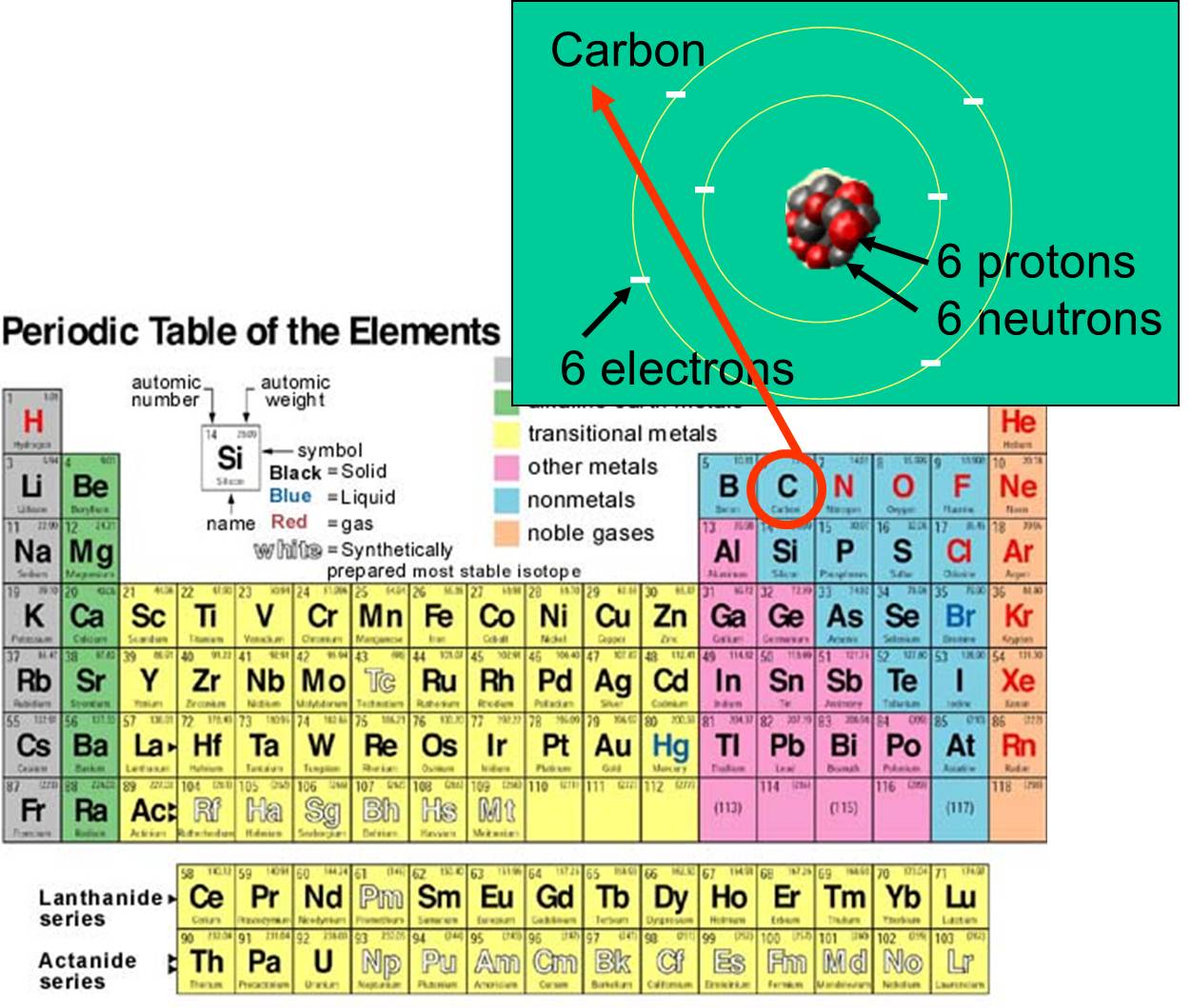
Periodic Table Of The Elements Definition Biology Elcho Table
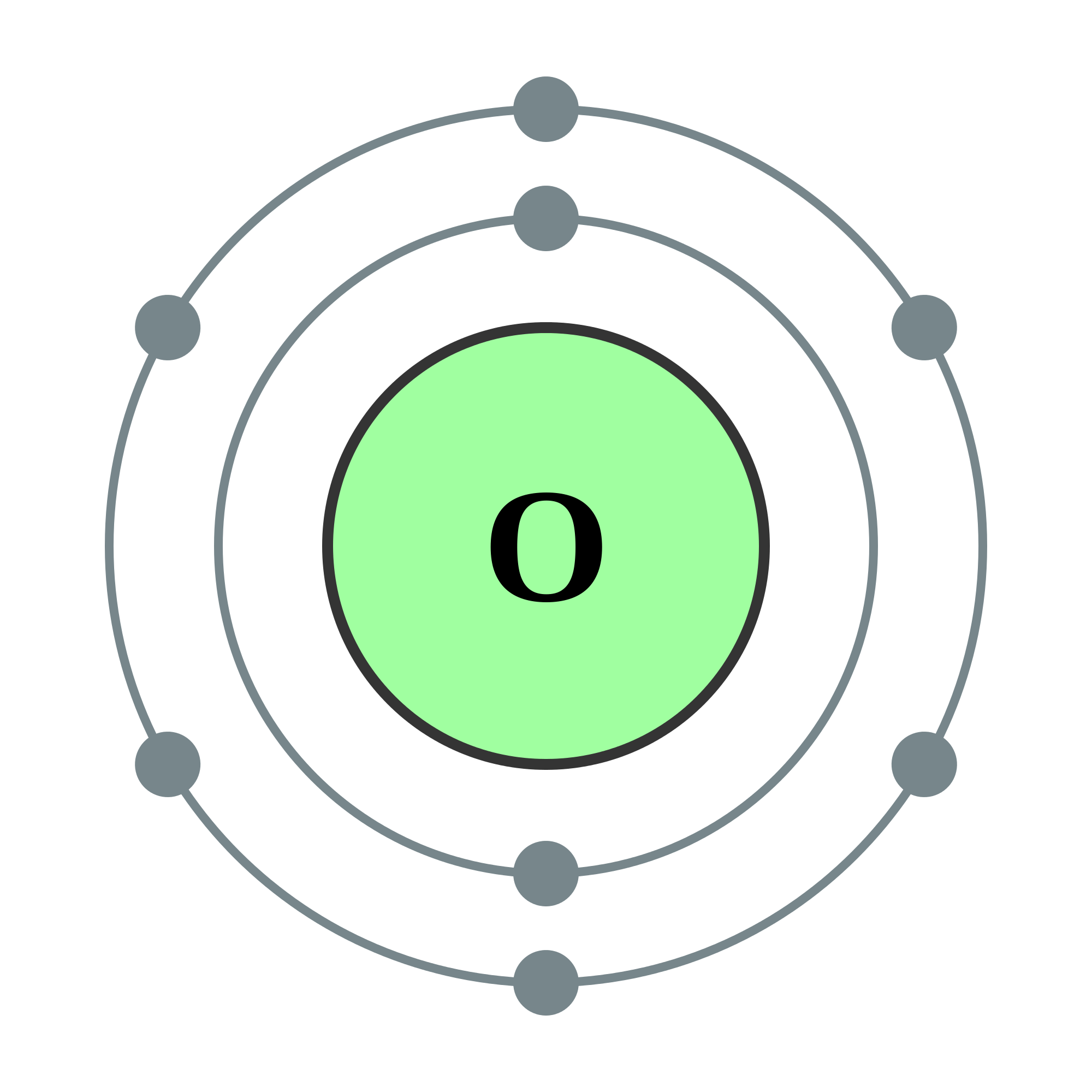
Electron Diagram For Oxygen

Start Spending Life With 8 8 8 Rule It Will Change Your Life YouTube

Start Spending Life With 8 8 8 Rule It Will Change Your Life YouTube

Mastering The 8 8 8 Rule A Guide To Balanced Living YouTube

THE 8 8 8 RULE How To Balance Your Life YouTube

8 8 8 Rule YouTube
2 8 8 Rule - [desc-14]