Classification Of Medical Devices Eu Important Information Canada has aligned the Workplace Hazardous Materials Information System WHMIS with the Globally Harmonized System of Classification and
What is a classification Classification is defined in Part 1 of the TDG Regulations as classification means for dangerous goods as applicable the shipping name the primary What is the fire tetrahedron To understand how to prevent fires it is important to know how a fire can occur
Classification Of Medical Devices Eu
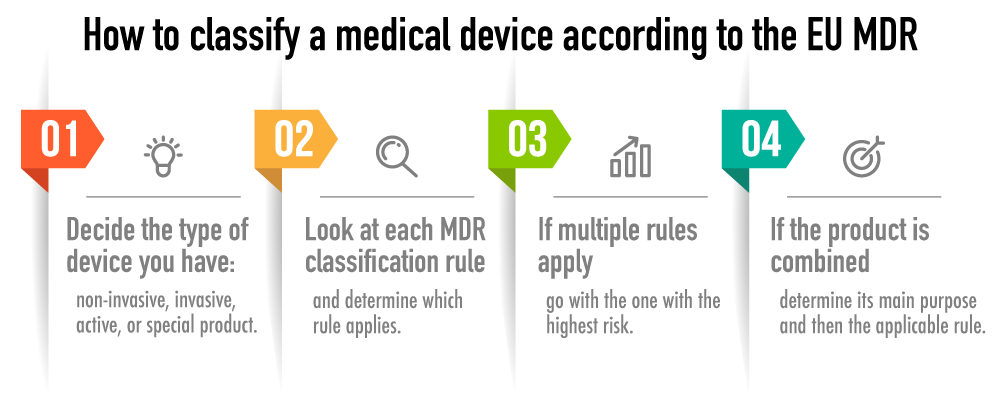
Classification Of Medical Devices Eu
https://advisera.com/wp-content/uploads/sites/14/2021/04/what-are-eu-mdr-classification-rules-for-medical-devices.png

Complete Guide Medical Device Classification EU MDR Free 51 OFF
https://easymedicaldevice.com/wp-content/uploads/elementor/thumbs/MedDev-Classes-1-npcezv6drndj9ovmld7opw6t4xw606sq0jl9k3fitc.jpg

Standards Certificates Green Back
https://www.presentationeze.com/wp-content/uploads/2014/10/FDA-medical-device-classification.jpg
The tertiary level of classification is species which is the most specific level and represents a group of organisms that can interbreed and produce fertile offspring MASS PACS
Asset classification is crucial for effective financial management Learn how to categorize assets into current non current tangible and intangible types Understand the Important Information Canada has aligned the Workplace Hazardous Materials Information System WHMIS with the Globally Harmonized System of Classification and
More picture related to Classification Of Medical Devices Eu

EU Medical Device Regulations APCER Life Sciences
https://www.apcerls.com/wp-content/uploads/2021/04/New_EU-MDR_1920X1080-1200x675.jpg
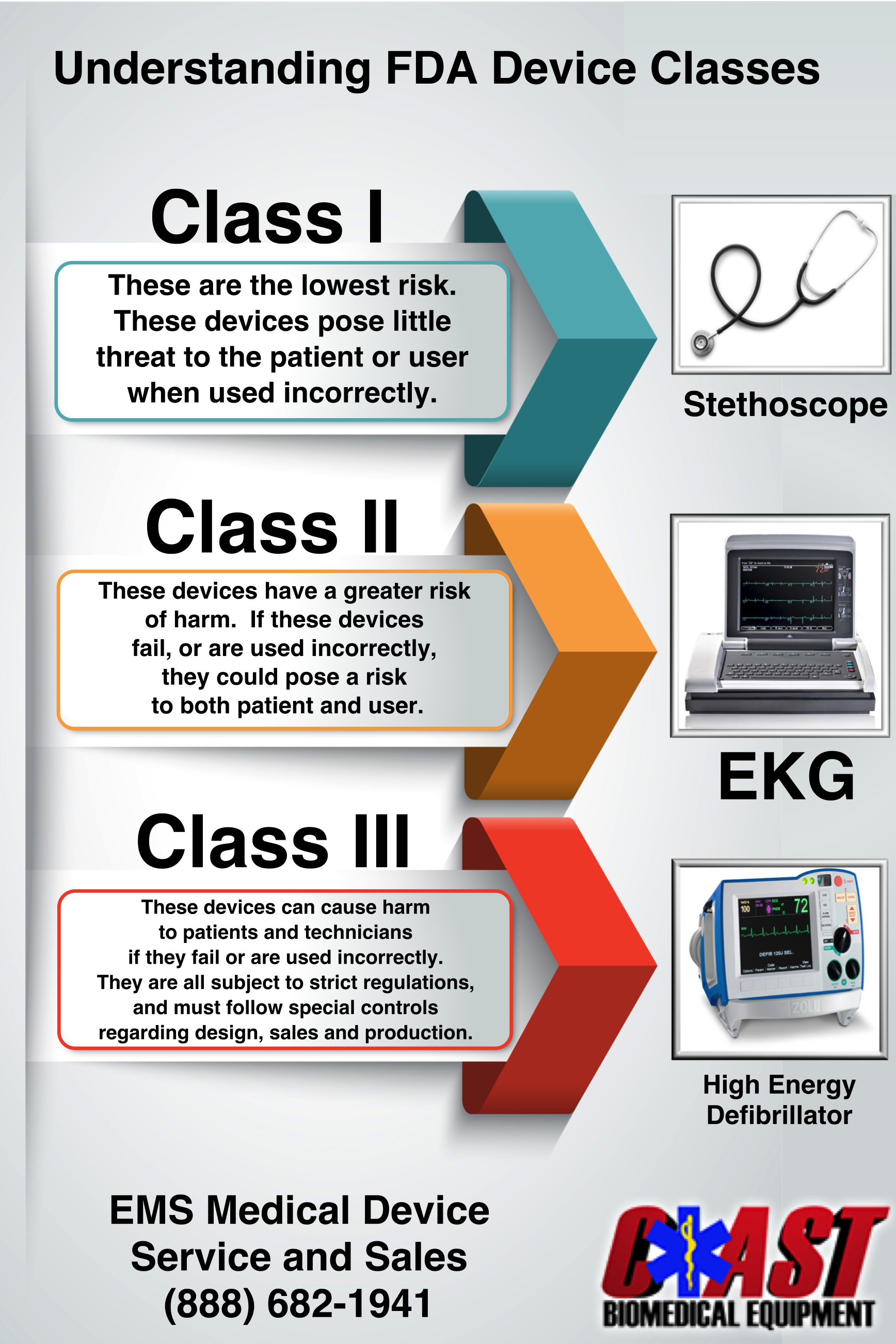
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment
https://coastbiomed.com/wp-content/uploads/2016/01/CoastInfographic.png
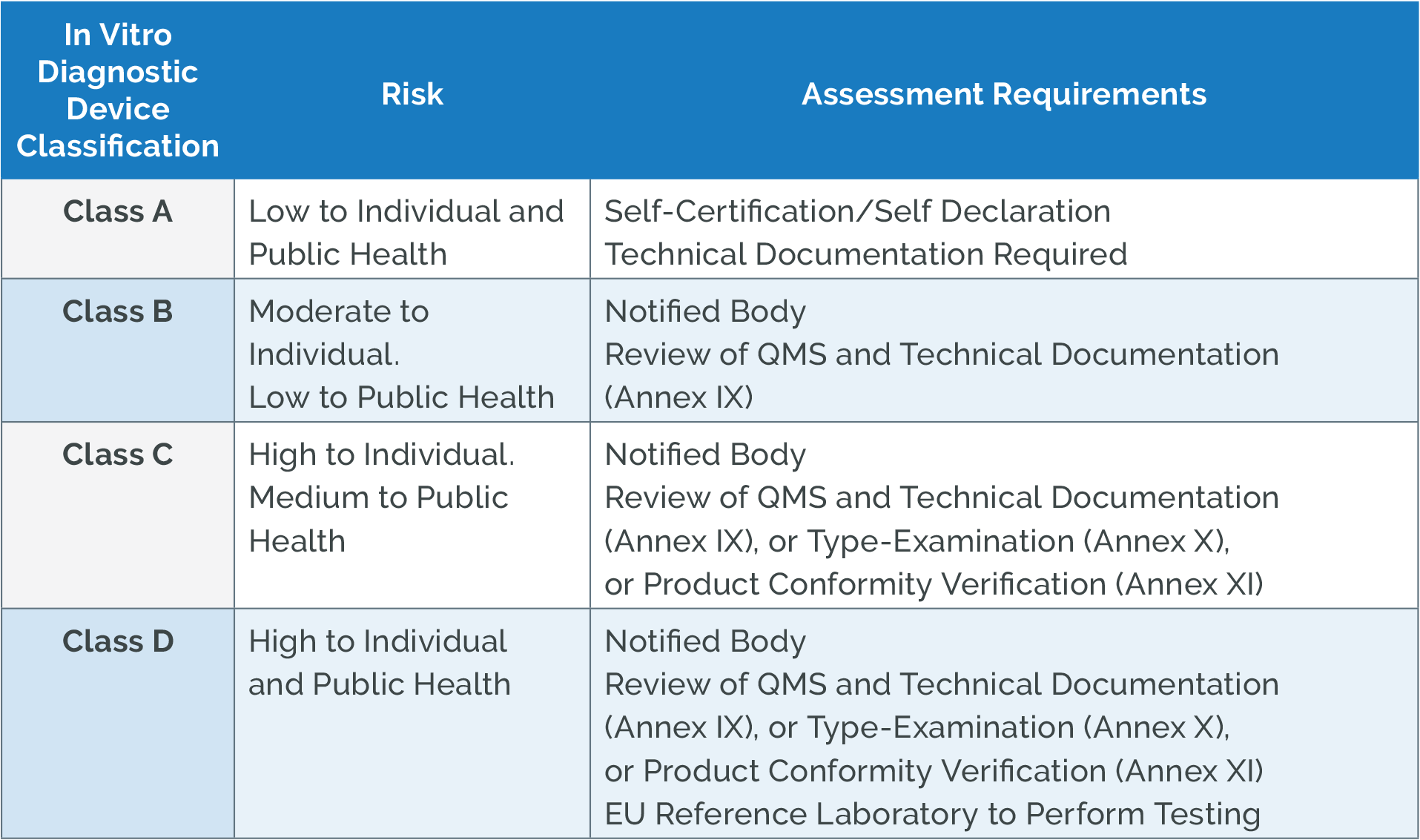
Key Changes Under New IVDR Arena
https://www.arenasolutions.com/wp-content/uploads/EU-IVDR-Class-Risk-Assessment-Requiremens.png
What are WHMIS classes or classifications WHMIS Workplace Hazardous Materials Information System uses classifications to group chemicals with similar properties What information is needed for classification Based on the definition for classification a competent person must determine the following before a classification can be assigned to a
[desc-10] [desc-11]
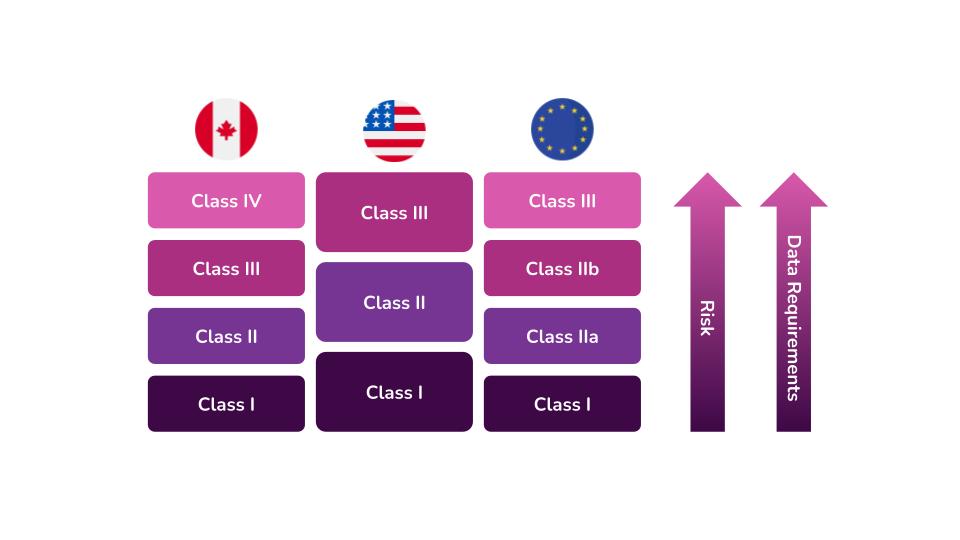
FDA Class II Medical Devices
https://assets-global.website-files.com/6141b0efd8e313f1168ba8cc/6321e274a9338e061e557731_Device class diagram.jpg

Ca Biomedical Devices
https://learn.marsdd.com/wp-content/uploads/2022/02/ClassII.png
https://www.ccohs.ca › oshanswers › chemicals › whmis_ghs › hazard_c…
Important Information Canada has aligned the Workplace Hazardous Materials Information System WHMIS with the Globally Harmonized System of Classification and

https://www.ccohs.ca › oshanswers › legisl › tdg › tdg_classification.html
What is a classification Classification is defined in Part 1 of the TDG Regulations as classification means for dangerous goods as applicable the shipping name the primary
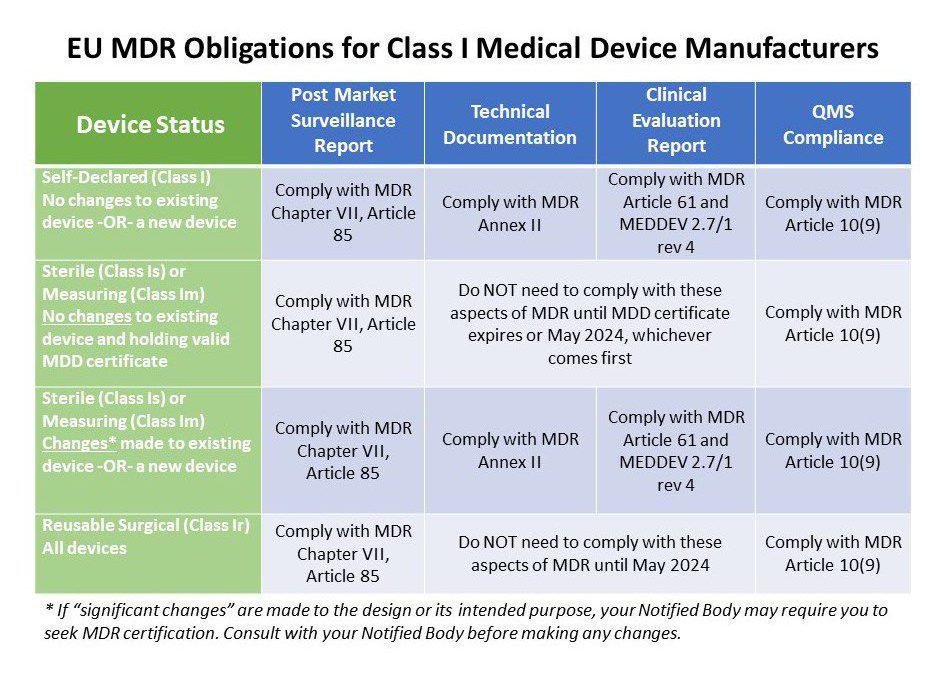
Class 1 Medical Device Requirements Oriel STAT A MATRIX

FDA Class II Medical Devices

Class 1 Medical Device Requirements Oriel STAT A MATRIX
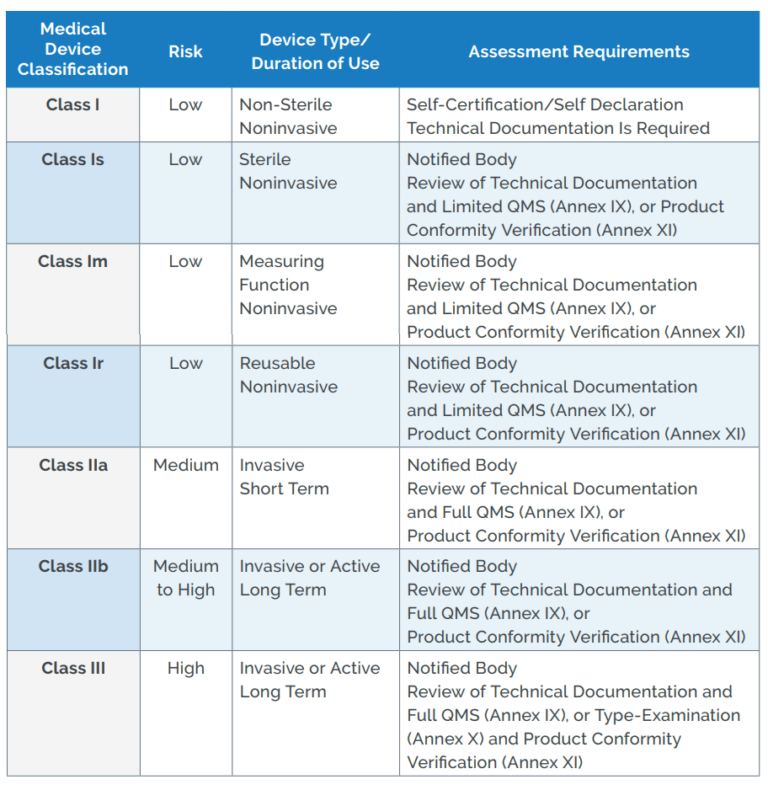
Classification Of Medical Devices Vrogue co
Key Changes Under New MDR Arena
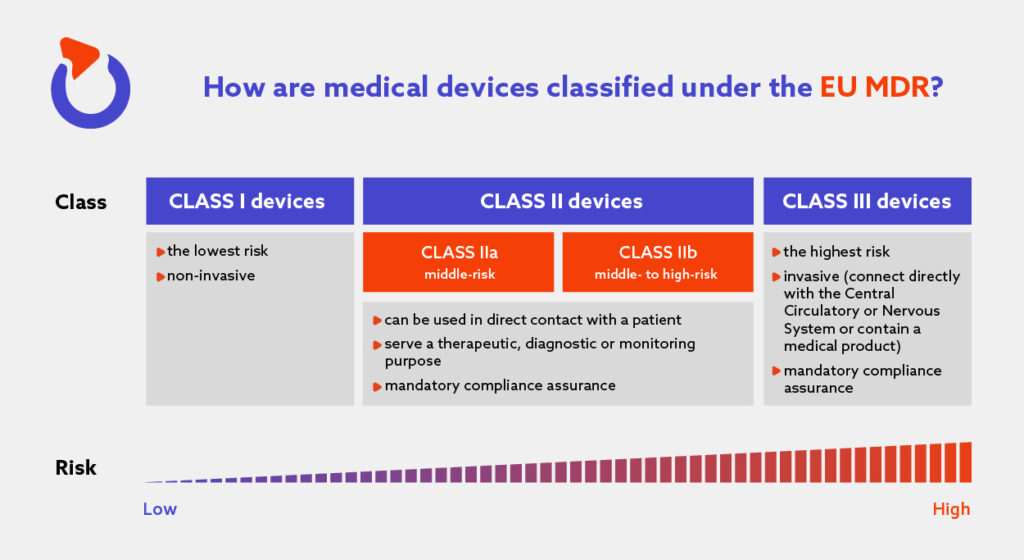
The Complete Guide To EU Medical Device Regulation Spyrosoft

The Complete Guide To EU Medical Device Regulation Spyrosoft

Device Classification In India Infographic Vrogue co

General Classification And Application Types Of Medical Devices For
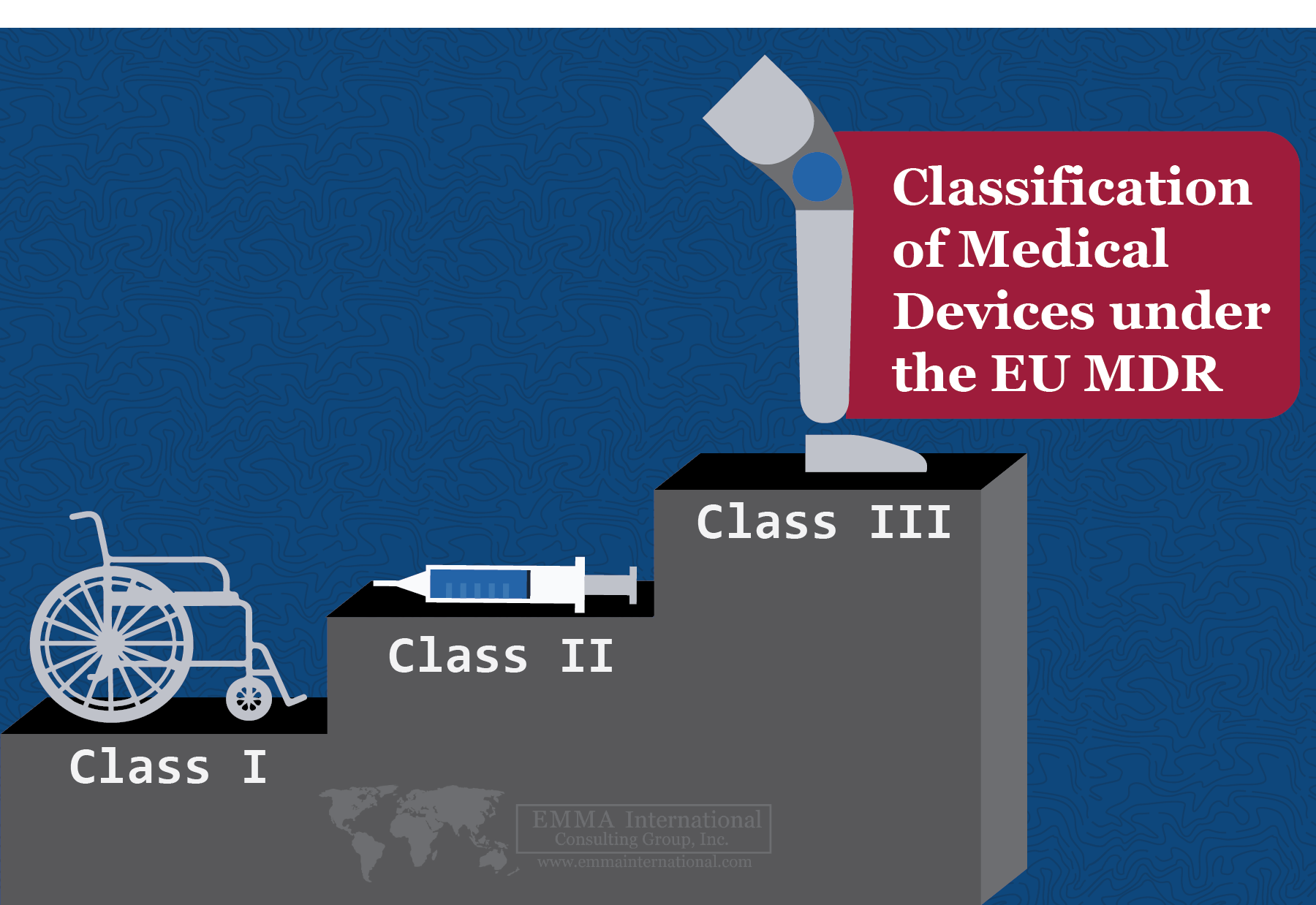
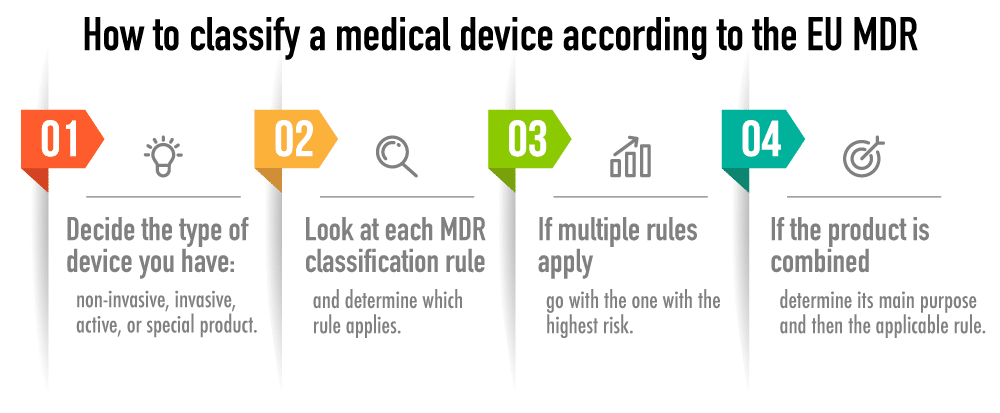
Classifying Medical Devices Under EU MDR
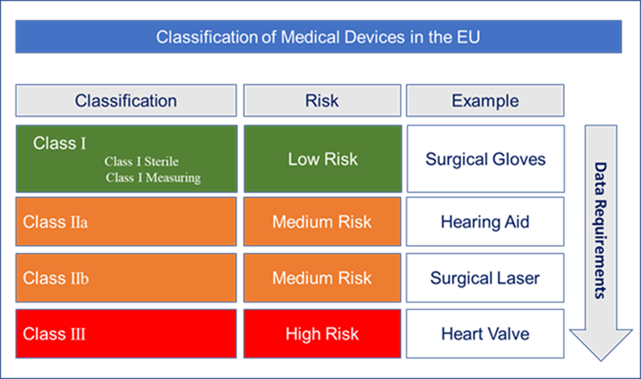
Classification Of Medical Devices Eu - The tertiary level of classification is species which is the most specific level and represents a group of organisms that can interbreed and produce fertile offspring