Dilute Acid Formula In Chemistry Learn about dilute acid its definition formula steps physical and chemical properties difference between concentrated and dilute acid and its uses
If you need to dilute acid start by working out how much water you will need to reach your desired result by This yields the most general form of the dilution equation C 1V 1 C 2V 2 To summarize C stands for the concentration of the solution V for the volume of the solution a subscript of 1 means before dilution and a subscript of 2 means
Dilute Acid Formula In Chemistry

Dilute Acid Formula In Chemistry
https://i.ytimg.com/vi/eVhJnFpRqG0/maxresdefault.jpg

How To Dilute An Acid Class 10 CBSE Chemistry Chapter 2 YouTube
https://i.ytimg.com/vi/Q0NwIp4Lv9E/maxresdefault.jpg

How To Write The Formula For Sulfuic Acid YouTube
https://i.ytimg.com/vi/SWeQUPuhG60/maxresdefault.jpg
Examples of dilute acids Dilute HCl dilute hydrochloric acid Hydrochloric acid is an aqueous solution of hydrogen chloride often known as muriatic acid The chemical formula or dilute hydrochloric acid formula is HCl Dilute acids can react with specific metals including magnesium and zinc to create a metal salt and hydrogen gas For instance when a diluted hydrochloric acid solution
Use our revision notes to learn what a dilute acid is for GCSE Chemistry Describe the difference between dilute and concentrated acids with diagrams A dilute acid is one in which the concentration of the water that has been mixed with the acid is greater than the concentration of the acid in its pure form For example sulfuric acid with a
More picture related to Dilute Acid Formula In Chemistry

Dilute Sulphuric Acid Test Chemistry Lab Manual YouTube
https://i.ytimg.com/vi/7vvlcYCK-E8/maxresdefault.jpg
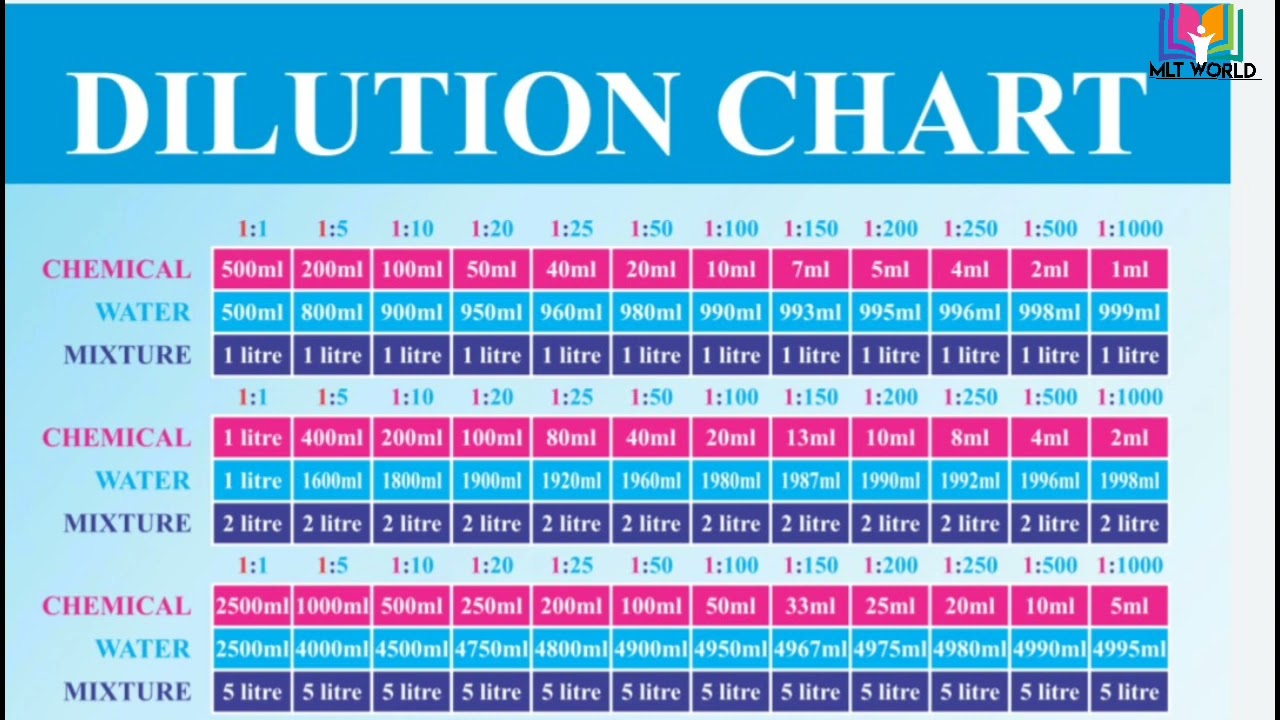
Dilution Chart Helpful Video Understand How To Prepare Dilutions In
https://i.ytimg.com/vi/vgg8wqNIpN4/maxresdefault.jpg
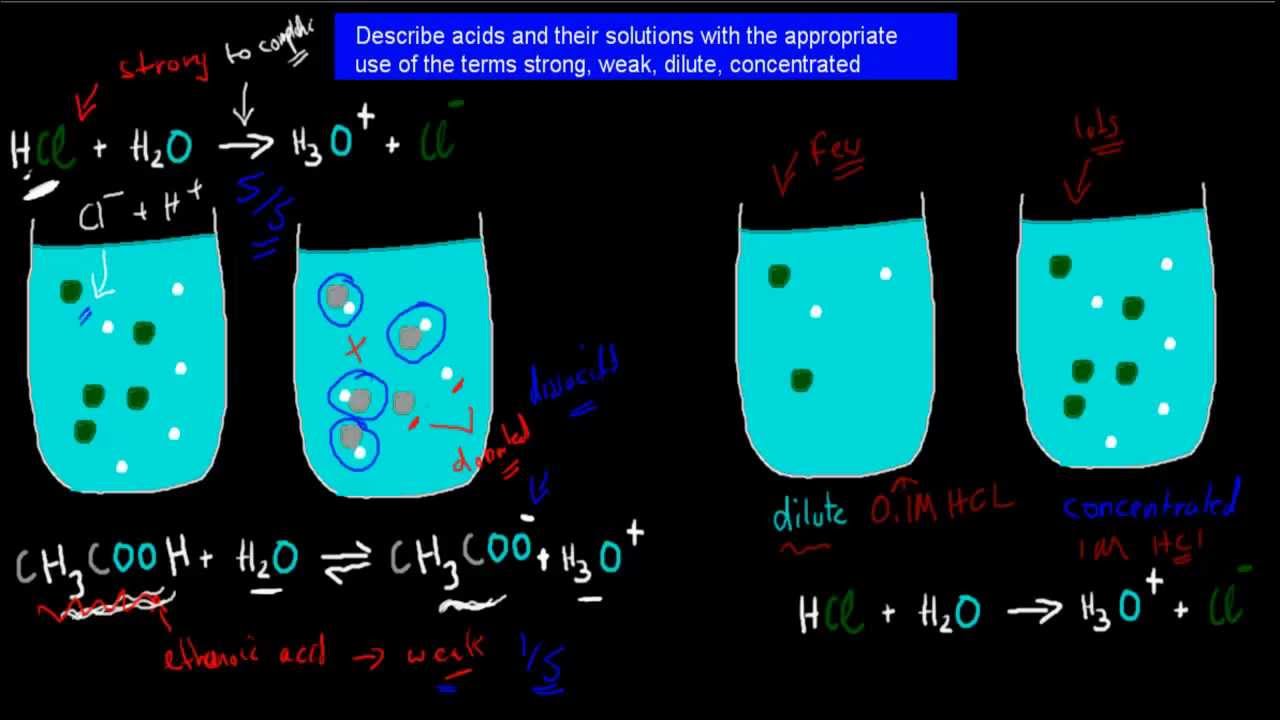
3 Strong Weak Dilute And Concentrated Acids HSC Chemistry YouTube
https://i.ytimg.com/vi/Y3hW3PolRHw/maxresdefault.jpg
Dilute Acid Dilute acids have high water content With the addition of water a concentrated acid can be diluted All acids whether organic or inorganic produce hydrogen What is Dilute Acid If an acid has been diluted it has lost more of its acidity to water than the water has gained from the acid It does not weaken acid or reduce its reactivity The acidity of the solution you re working with will decrease
Dilution is the process of reducing the concentration of a given solute in its solution The chemist can do it simply by mixing with more solvent In this topic the student will learn and understand the Dilution Formula with examples Muriatic acid another name for ce HCl is widely used for cleaning concrete and masonry surfaces The acid must be diluted before use to get it down to a safer strength Commercially

Reaction Of Metal With Dilute Acid Chemical Properties Hindi YouTube
https://i.ytimg.com/vi/ngzb0AQn9ro/maxresdefault.jpg

What Is The Difference Between A Concentrated Solution And A Dilute
https://i.ytimg.com/vi/UoeKOvrzuzs/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLDH1okbXdtLVNygJBKjWQJKsao2wQ

https://testbook.com › chemistry › dilute-acid
Learn about dilute acid its definition formula steps physical and chemical properties difference between concentrated and dilute acid and its uses

https://www.wikihow.com › Dilute-an-Acid
If you need to dilute acid start by working out how much water you will need to reach your desired result by

Explaining The Electrolysis Of Dilute Sulfuric Acid H2SO4 aq GCSE

Reaction Of Metal With Dilute Acid Chemical Properties Hindi YouTube

Reaction Of Zinc Granules With Dilute Sulphuric Acid And Testing
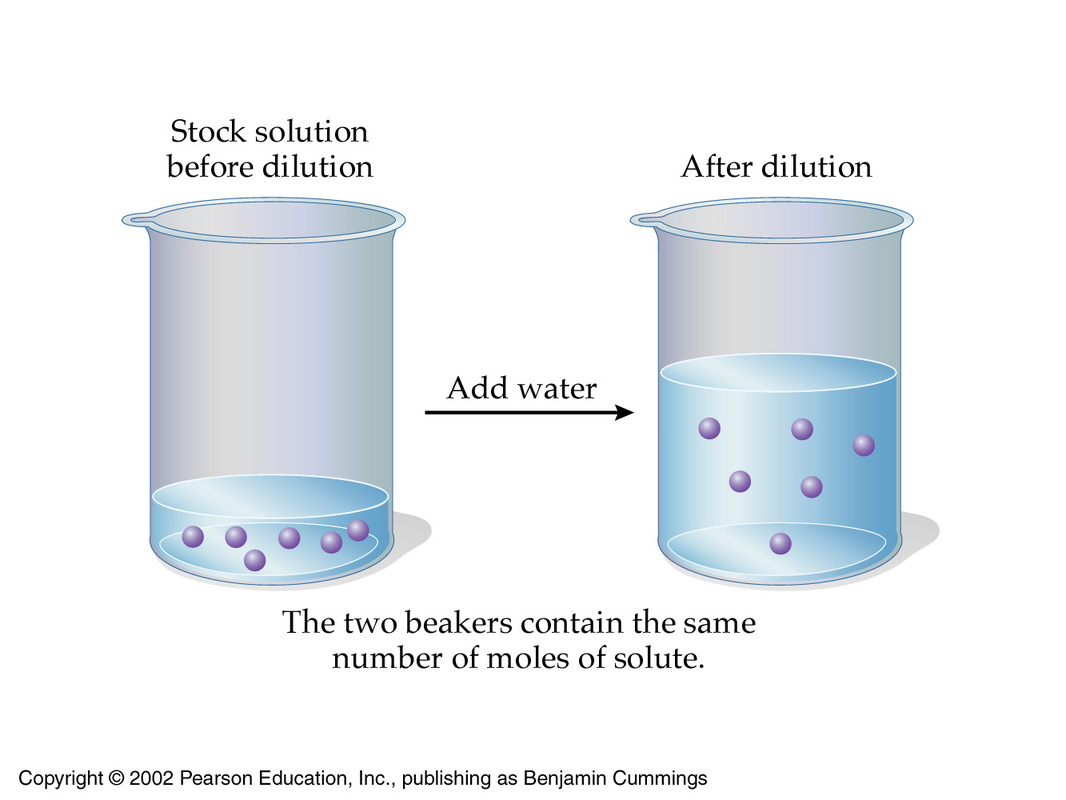
Molarity Acid And Bases For Dummies
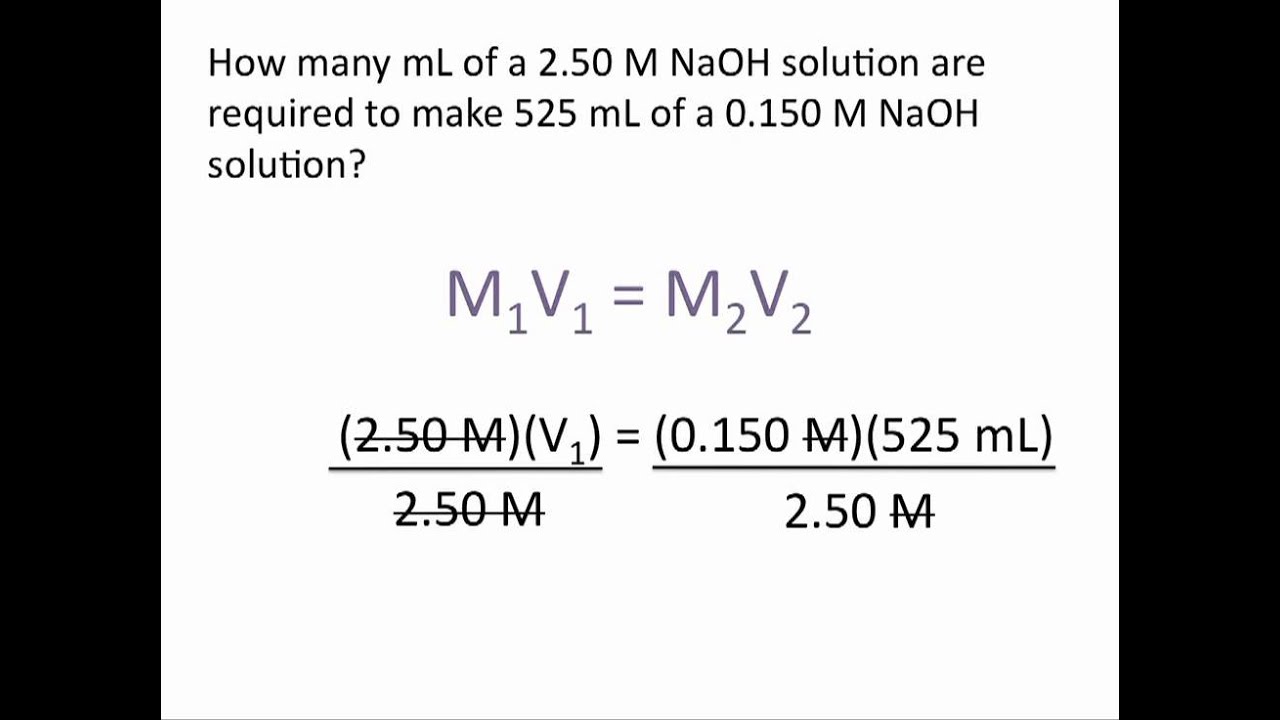
Dilution Problems Chemistry Tutorial YouTube

Write A Balanced Equation For Dilute Sulphuric Acid Is Poured Over

Write A Balanced Equation For Dilute Sulphuric Acid Is Poured Over

Acetic Acid Dilute 500ml MedicoLab

Pre AP Chemistry Dilutions
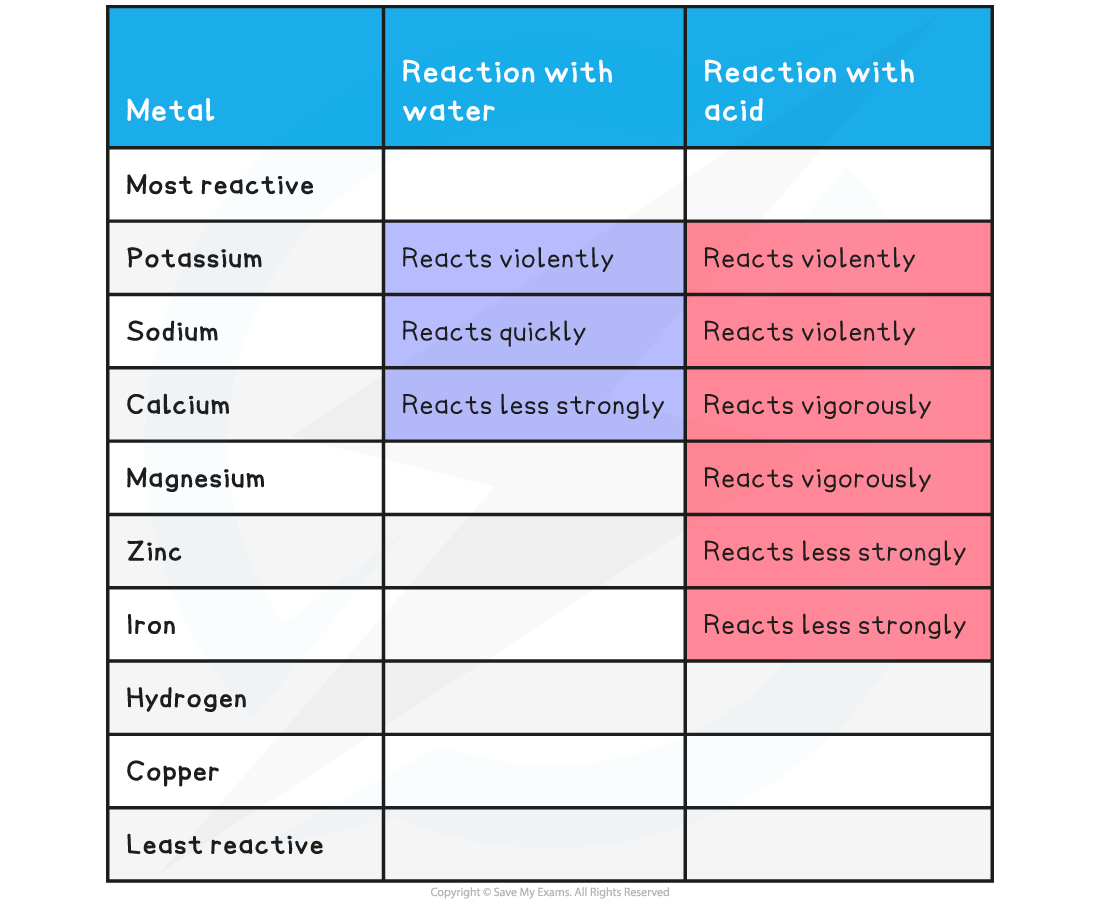
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 2 4 1 Metals Reacting With
Dilute Acid Formula In Chemistry - When solutions are described as dilute or concentrated dissolve When a substance breaks up and mixes completely with a solvent to produce a solution Take care to use the word dilute