Dilute Hydrochloric Acid Dilute Hydrochloric Acid Dil HCl is a weakened aqueous solution of hydrochloric acid HCl used in medicine digestion support and industrial applications It plays a vital role as a gastric acidifier aiding in digestion metabolic regulation and pharmaceutical formulations
Dilute acids contain a large amount of water A concentrated acid can be diluted with the addition of water All acids may be an organic or inorganic substance which releases hydrogen ions H in water Hence acid is defined as a substance which gives To neutralise concentrated acid first dilute the acid by adding it carefully to a larger volume of water Neutralise waste by addition of sodium bicarbonate until no further fizzing occurs so that the pH is in the range pH 6 8 Use an indicator such as Universal Indicator to determine pH
Dilute Hydrochloric Acid

Dilute Hydrochloric Acid
https://us-static.z-dn.net/files/d1e/b8d131ba3f368eecff9bdf71a01c6898.png

Dilute Hydrochloric Acid Edu svet gob gt
https://www.jaincolab.com/images/catalog/product/1227298003HydrochloricAcidDilute.jpg

Dilute Hydrochloric Acid Edu svet gob gt
https://o.quizlet.com/0qGuiMYY7LejK0z00Njf.g_b.png
How to Dilute Acids Before you perform the dilution itself calculate the amount of water and acid needed for the desired concentration For example to make 100 mL of 01 molar M hydrochloric acid use 10 mL of 1 molar acid and 90 mL of water Obtain the correct amounts of deionized DI water in one beaker and acid in another Dilute Hydrochloric Acid can be prepared by diluting concentrated hydrochloric acid HCl with water The concentration of the resulting solution depends on the ratio of acid to water Typically laboratory grade dilute hydrochloric acid has a concentration of around 3 10
Assay Transfer 10 0 mL of Diluted Hydrochloric Acid to a conical flask and add about 20 mL of water Add 3 drops of methyl red TS and titrate with 1 N sodium hydroxide VS Each mL of 1 N sodium hydroxide is equivalent to 36 46 mg of HCl Step 2 Calculate how much stock solution is required to prepare 1L of 1M hydrochloric acid solution using the dilution method Dilution method is described in detail in the post Preparing Working Solution from Stock Solution by Dilution
More picture related to Dilute Hydrochloric Acid

Dilute Hydrochloric Acid Reactions Stock Image A500 0552 Science
https://media.sciencephoto.com/image/a5000552/800wm/A5000552-Dilute_hydrochloric_acid_reactions.jpg

Magnesium And Dilute Hydrochloric Acid
https://c8.alamy.com/comp/B06106/reactivity-of-metals-magnesium-zinc-iron-and-lead-in-dilute-hydrochloric-B06106.jpg

Dilute Hydrochloric Acid For Laboratory 99 Pure At Rs 300 litre In
https://5.imimg.com/data5/SELLER/Default/2022/9/HV/ST/ZP/152317913/dilute-hydrochloric-acid-500x500.jpg
Dilute Hydrochloric Acid Prepared by carefully adding concentrated hydrochloric acid HCl to water in a volumetric flask It is crucial to add acid to water slowly to prevent excessive heat release and splashing Colorless to slightly yellow liquid with a strong pungent odor To prepare dilute hydrochloric acid HCl you can follow these steps 1 Start with concentrated hydrochloric acid usually around 37 concentration 2 Use a clean and dry glass container 3 Add the desired amount of concentrated HCl to the container 4 Carefully add distilled water to the acid while stirring constantly 5
[desc-10] [desc-11]
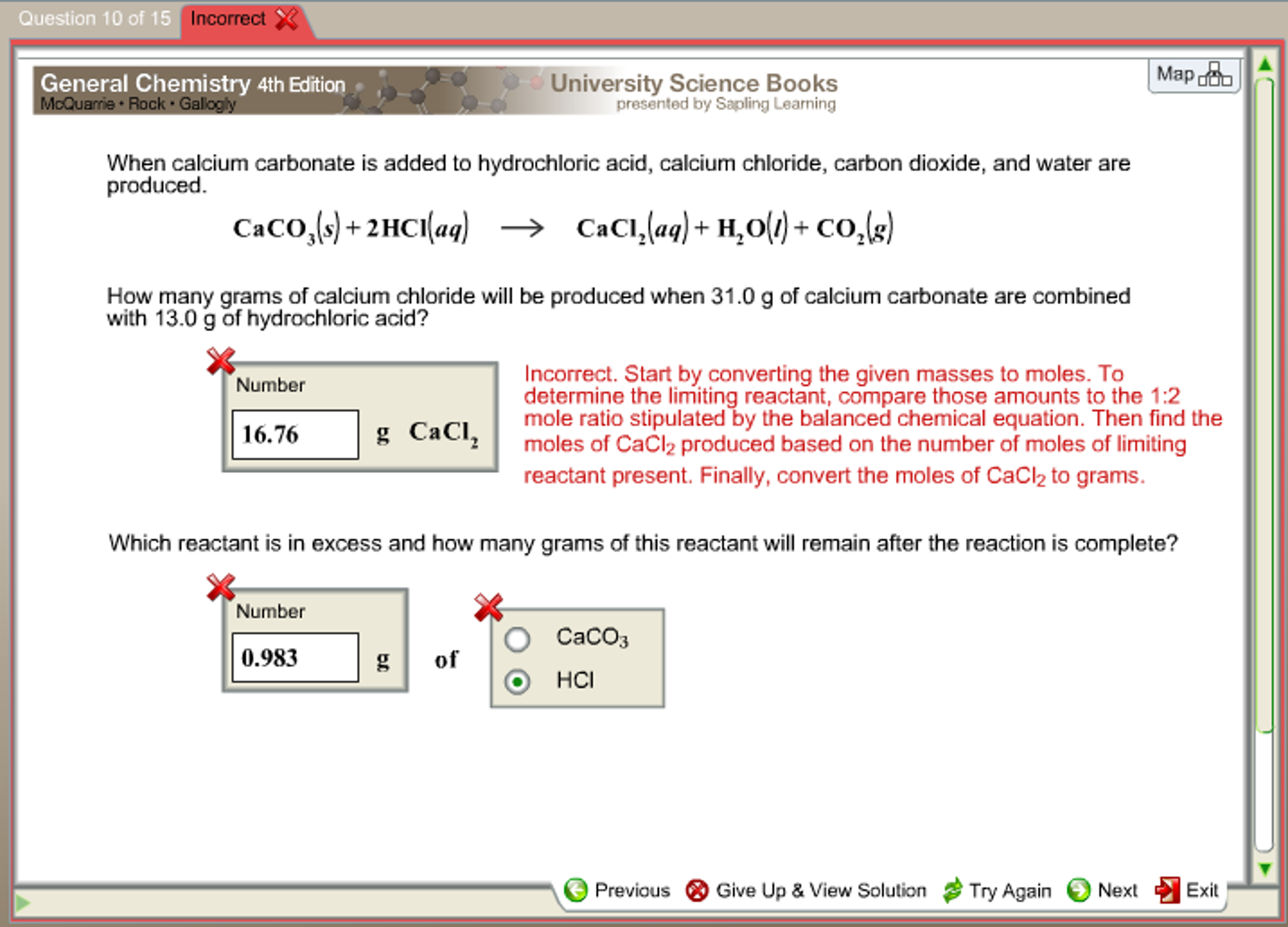
Calcium Carbonate And Dilute Hydrochloric Acid
https://d2vlcm61l7u1fs.cloudfront.net/media/45b/45b7338f-8012-42e2-8b9b-dec690f7079e/phpaRt4Gc.png

Bottle Of Dilute Hydrochloric Acid Stock Image C019 8337 Science
https://media.sciencephoto.com/image/c0198337/800wm/C0198337-Bottle_of_dilute_hydrochloric_acid.jpg

https://www.pharmacareerinsider.com › dilute...
Dilute Hydrochloric Acid Dil HCl is a weakened aqueous solution of hydrochloric acid HCl used in medicine digestion support and industrial applications It plays a vital role as a gastric acidifier aiding in digestion metabolic regulation and pharmaceutical formulations

https://byjus.com › chemistry › dilute-acids
Dilute acids contain a large amount of water A concentrated acid can be diluted with the addition of water All acids may be an organic or inorganic substance which releases hydrogen ions H in water Hence acid is defined as a substance which gives

Bottle Of Dilute Hydrochloric Acid Stock Image C019 8335 Science

Calcium Carbonate And Dilute Hydrochloric Acid

Calcium Carbonate Reacts With Dilute Hydrochloric Acid To Produce Stock

Calcium Carbonate Reacts With Dilute Hydrochloric Acid To Produce Stock

Explain The Action Of Dilute Hydrochloric Acid On The Following
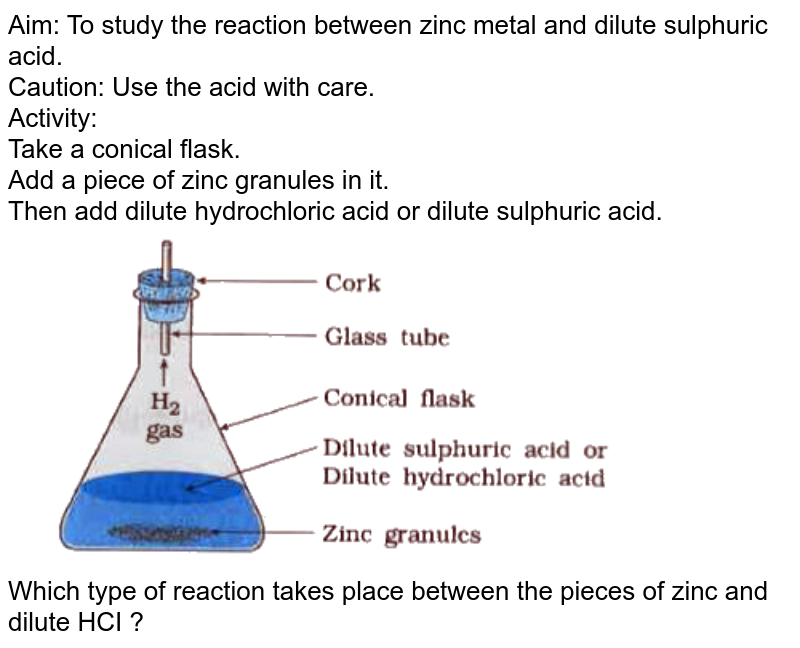
Chemical Tests Between Dilute Hydrochloric Acid And Dilute Sulphur

Chemical Tests Between Dilute Hydrochloric Acid And Dilute Sulphur

Keerti Added Dilute Hydrochloric Acid To Four Metals And Recorded Her Obs
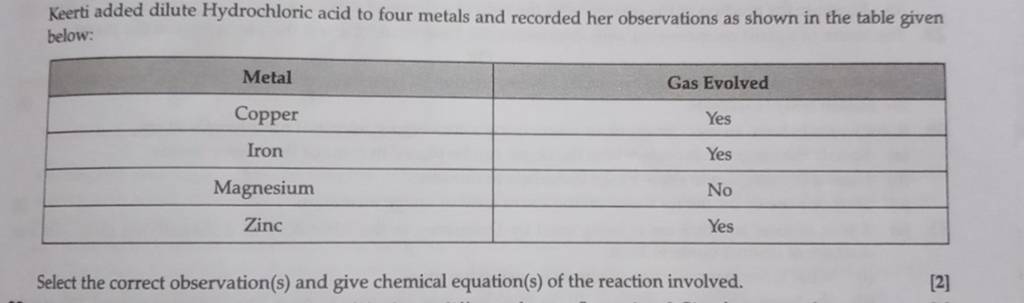
Keerti Added Dilute Hydrochloric Acid To Four Metals And Recorded Her Obs

3 Metal Compound A Reacts With Dilute Hydrochloric Acid To Produce Effer
Dilute Hydrochloric Acid - Assay Transfer 10 0 mL of Diluted Hydrochloric Acid to a conical flask and add about 20 mL of water Add 3 drops of methyl red TS and titrate with 1 N sodium hydroxide VS Each mL of 1 N sodium hydroxide is equivalent to 36 46 mg of HCl