Dilution Of Sulphuric Acid Equation Dilution is the addition of solvent which decreases the concentration of the solute in the solution Concentration is the removal of solvent which increases the concentration of the solute in the
Dilution is the process of decreasing the concentration of a solute in a solution usually simply by mixing with more solvent like adding more water to the solution Dilution is a fundamental concept in chemistry that involves reducing the concentration of a substance in a solution by adding a solvent It is a technique that is used to create less
Dilution Of Sulphuric Acid Equation

Dilution Of Sulphuric Acid Equation
https://i.ytimg.com/vi/BxB3DGV8Kyg/maxresdefault.jpg

Chem Resist Sulphuric Acid Dilution Systems YouTube
https://i.ytimg.com/vi/LlcKW47fdH0/maxresdefault.jpg
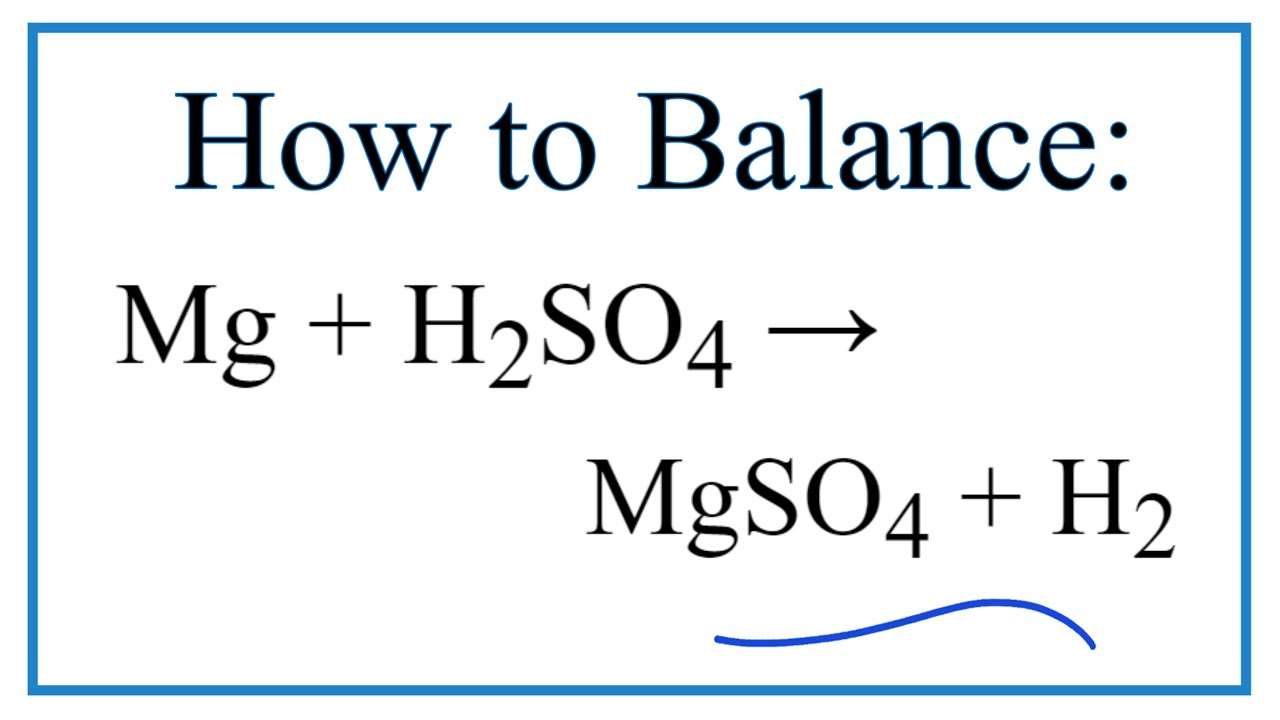
How To Balance Mg H2SO4 MgSO4 H2 Magnesium Dilute Sulfuric
https://i.ytimg.com/vi/bvOXULLt7QQ/maxresdefault.jpg
The act of reducing the concentration of a solution by adding additional solvent is known as dilution Dilution involves adding additional solvent to a sample of a solution The process does The dilution is by a factor of 32 to go from 16 M to 0 5 M Image 1 Volumetric pipette Image from the Teaching and Learning Centre Figure 2 Micropipette Image by rocksee Dilutions can be
Definition Dilution is the act of adding a solvent usually water to a solution What is dilution Dilution is when you add a solvent to a solution In A level Chemistry dilution only occurs with Another common dilution problem involves calculating what amount of a highly concentrated solution is required to make a desired quantity of solution of lesser concentration
More picture related to Dilution Of Sulphuric Acid Equation

Reaction Between Zinc And Dilute Sulphuric Acid Formation Of Hydrogen
https://i.ytimg.com/vi/28mJ0U6bu8Y/maxresdefault.jpg

Explaining The Electrolysis Of Dilute Sulfuric Acid H2SO4 aq GCSE
https://i.ytimg.com/vi/fbGr7bKVg5o/maxresdefault.jpg

SOLVED Equation For The Reaction Benzaldehyde Between 49 OFF
https://unacademy.com/content/wp-content/uploads/sites/2/2022/03/st1-1.png
Dilution is the process of decreasing the concentration of solute in a solution by changing the amount of solvent The dilute solution definition requires an understanding of Dilution is the process of reducing the concentration of a solute in solution usually simply by mixing with more solvent Example 1 You can add water to concentrated orange juice to dilute
[desc-10] [desc-11]

Write A Balanced Equation For Dilute Sulphuric Acid Is Poured Over
https://hi-static.z-dn.net/files/d57/b8059e77cd6f07b7feb96595df0537f3.jpg

Concentrated Sulfuric Acid
http://www.inyoprocess.com/images/chem_appl/heat of enthalpy chart.jpg

https://chem.libretexts.org › Bookshelves...
Dilution is the addition of solvent which decreases the concentration of the solute in the solution Concentration is the removal of solvent which increases the concentration of the solute in the

https://en.wikipedia.org › wiki › Dilution_(equation)
Dilution is the process of decreasing the concentration of a solute in a solution usually simply by mixing with more solvent like adding more water to the solution
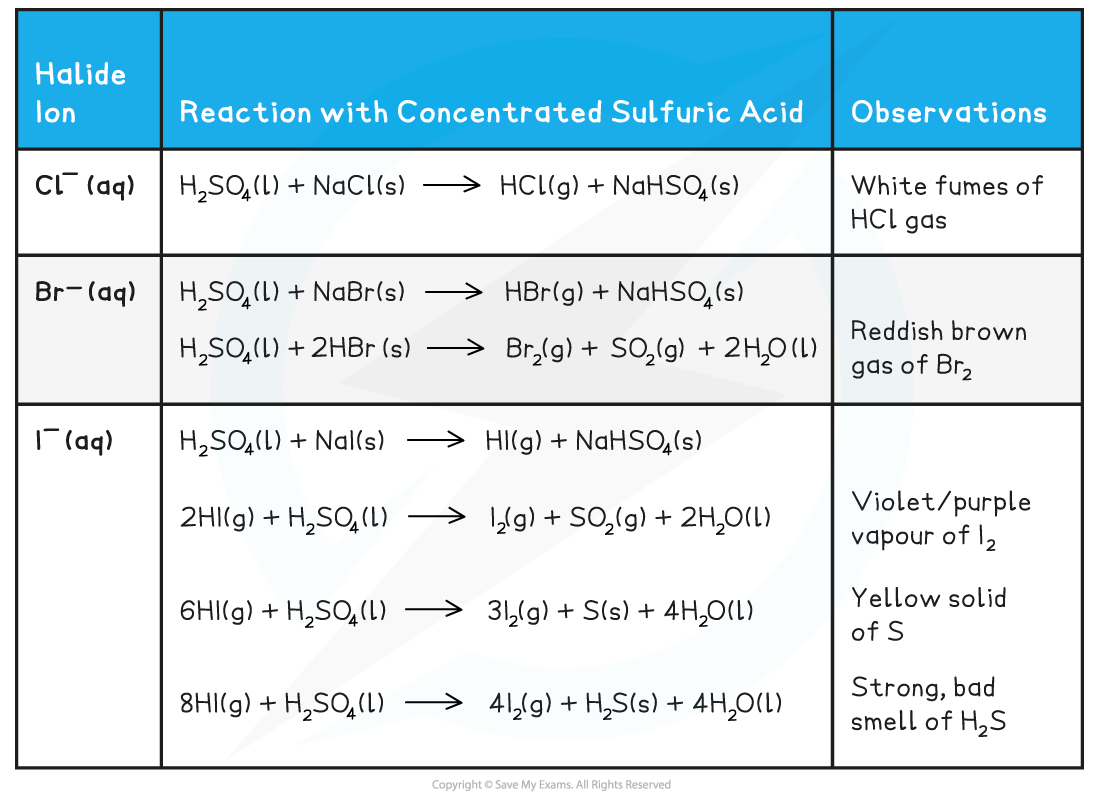
Edexcel A Level Chemistry 2 3 4 Halide Ion Reactions

Write A Balanced Equation For Dilute Sulphuric Acid Is Poured Over
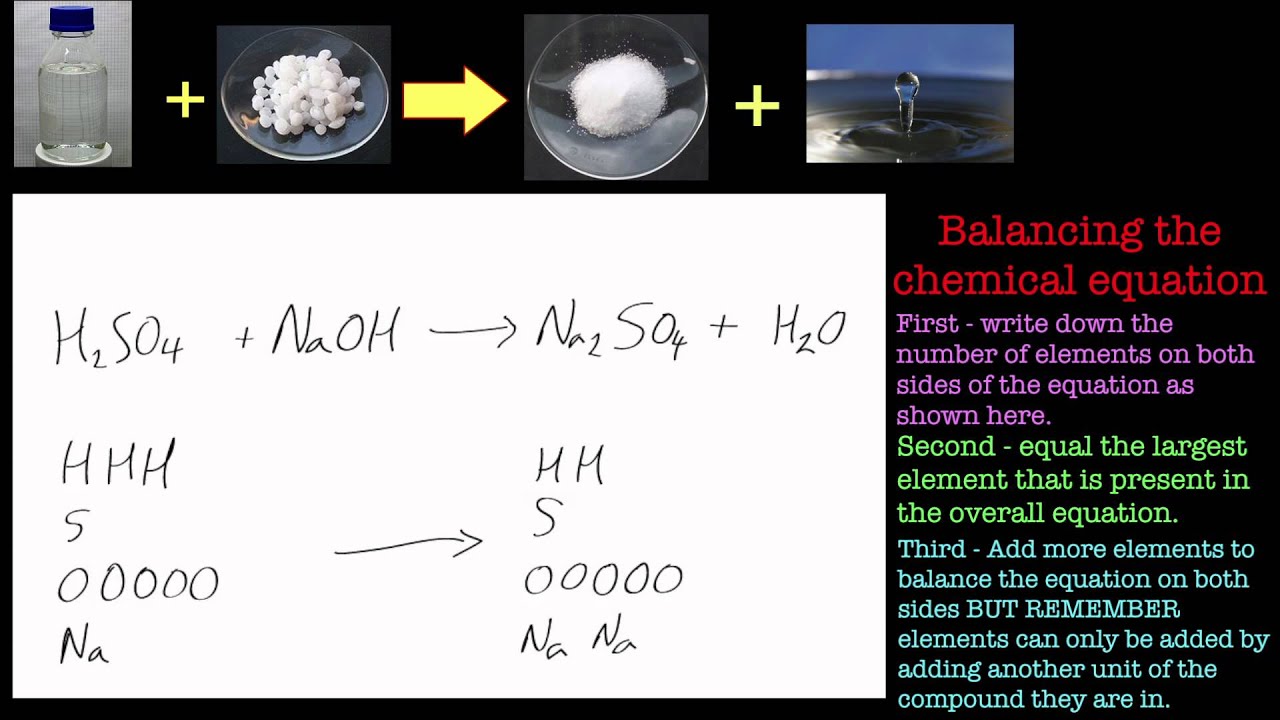
Balancing Chemical Equations Part 2 Sulfuric Acid And Sodium

Question Video Calculating The Volume Of Sulfuric Acid That Completely

Sulphuric Acid Chemical Formula On Waterdrop Background Stock

Chemical Acid Reaction

Chemical Acid Reaction

Sulfuric Acid Formation

H2so4 Acid

Balanced Equation Of Sucrose And Sulfuric Acid Tessshebaylo
Dilution Of Sulphuric Acid Equation - [desc-13]