How Tall Is 39 1 2 Inches Working at our GMP Licenced CDMO facility in Keele you will be collaborating with Operations Quality Assurance and Quality Control teams to perform activities under the site Validation
The QC Method Validation Specialist role is integral to our GMP operations and among other things will perform tasks associated with stability programs purity method development CQV is essential to Ensure Compliance GMP regulations e g FDA s 21 CFR Parts 210 211 EU GMP Annex 15 require facilities to prove that equipment systems and processes are
How Tall Is 39 1 2 Inches

How Tall Is 39 1 2 Inches
https://i.ytimg.com/vi/XOpMNcIpO4Q/maxresdefault.jpg
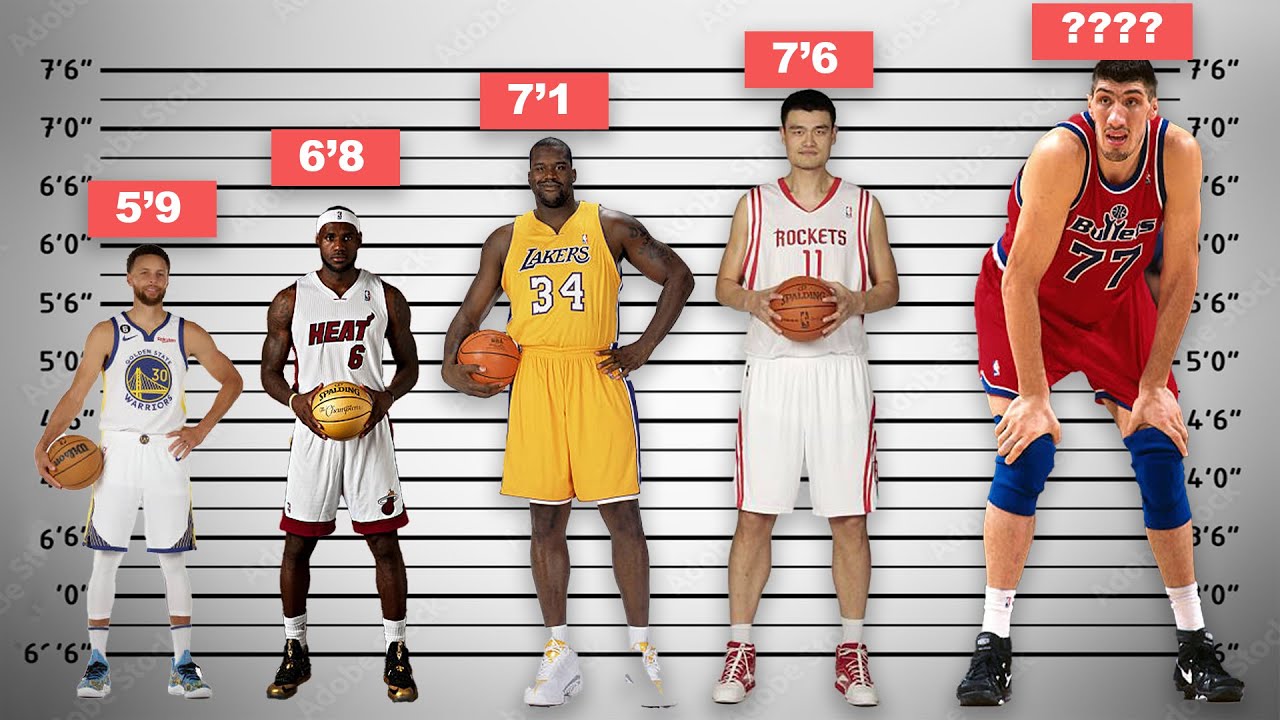
NBA Star Players Height Comparison NBA Heights Smallest To Tallest
https://i.ytimg.com/vi/bGFbQ20NZG0/maxresdefault.jpg

I Spent 24 Hours With The World s Tallest Man YouTube
https://i.ytimg.com/vi/HipdY8pWJ2k/maxresdefault.jpg
In Good Manufacturing Practices GMP both validation and qualification are critical processes that ensure pharmaceutical products meet quality safety and efficacy What Certification do we offer What is GMP Get the GMP App
Qualification is objective evidence that equipment or system and its ancillary systems are correctly installed work as expected and fit for the intended use Validation is the Chemical Engineer with hands on experience in GMP compliance system validation and quality assurance within the pharmaceutical sector Proven track record in executing validation
More picture related to How Tall Is 39 1 2 Inches

2 Ways To Convert Inches To Feet inches YouTube
https://i.ytimg.com/vi/IjtuSAklsRo/maxresdefault.jpg

Ground Launch Small Diameter Bomb GBU39 HIMARS MLRS How It Works
https://i.ytimg.com/vi/slwQZIlxAP8/maxresdefault.jpg

Exploring California s Highway 39 Closed For Over 40 Years 57 OFF
https://i.ytimg.com/vi/a5mtZNru7BA/maxresdefault.jpg
The Quality Control QC department plays a crucial role in ensuring Good Manufacturing Practices GMP compliance within pharmaceutical and medical device Comparison between Qualification and Validation Qualification is normally used for equipment utilities and systems and Validation is normally used for processes and
[desc-10] [desc-11]
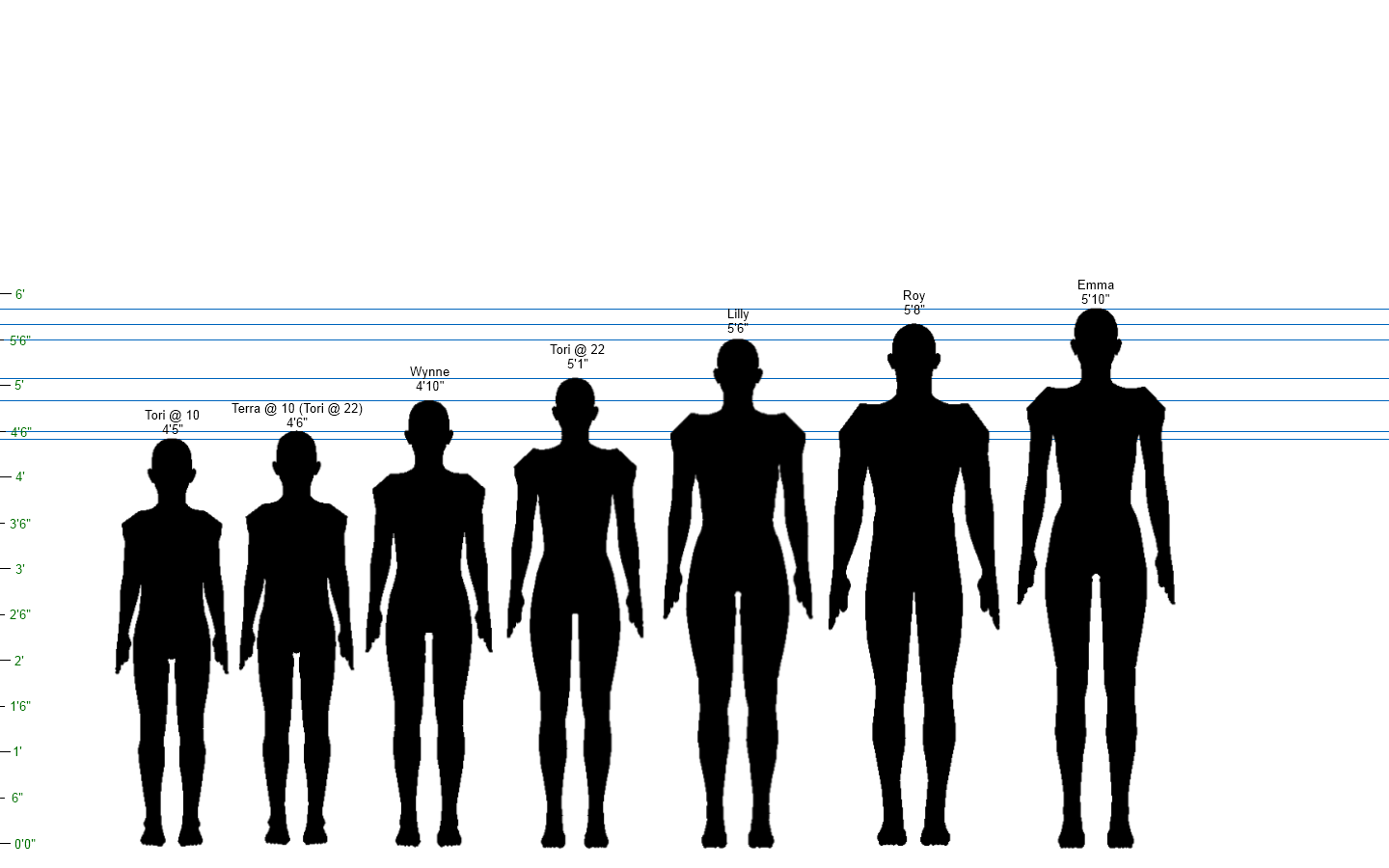
https://englishbookgeorgia.com/blogebg/wp-content/uploads/2015/01/shorter-in-the-evening.png
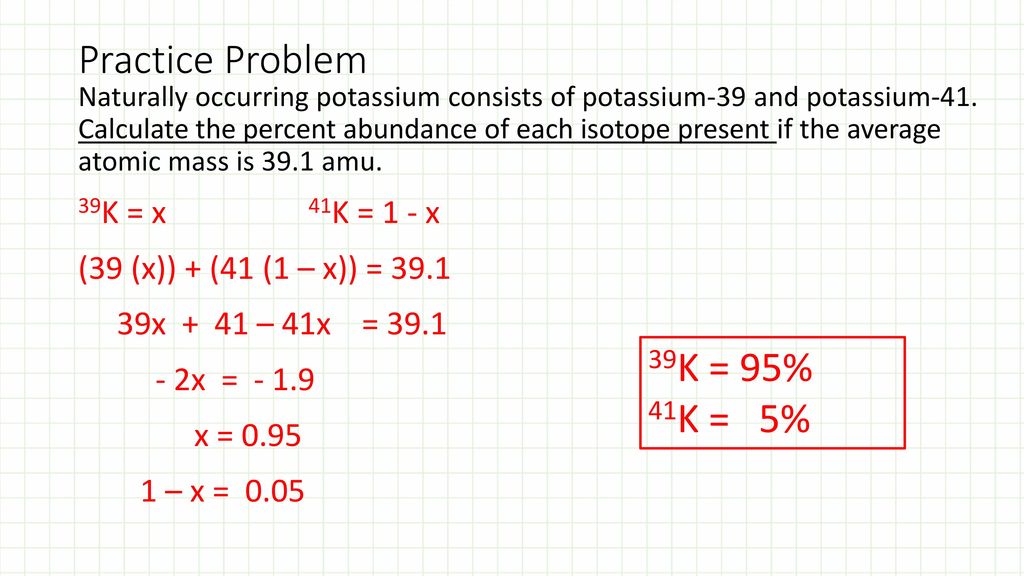
Mass Spectrometry Ppt Download
https://slideplayer.com/slide/13195014/79/images/14/Practice+Problem+39K+%3D+95%25+41K+%3D+5%25+39K+%3D+x+41K+%3D+1+-+x.jpg

https://careers.criver.com › job
Working at our GMP Licenced CDMO facility in Keele you will be collaborating with Operations Quality Assurance and Quality Control teams to perform activities under the site Validation

https://www.anaspec.com › en › jobs › job › details
The QC Method Validation Specialist role is integral to our GMP operations and among other things will perform tasks associated with stability programs purity method development

Frank Fiola Height CelebsHeight

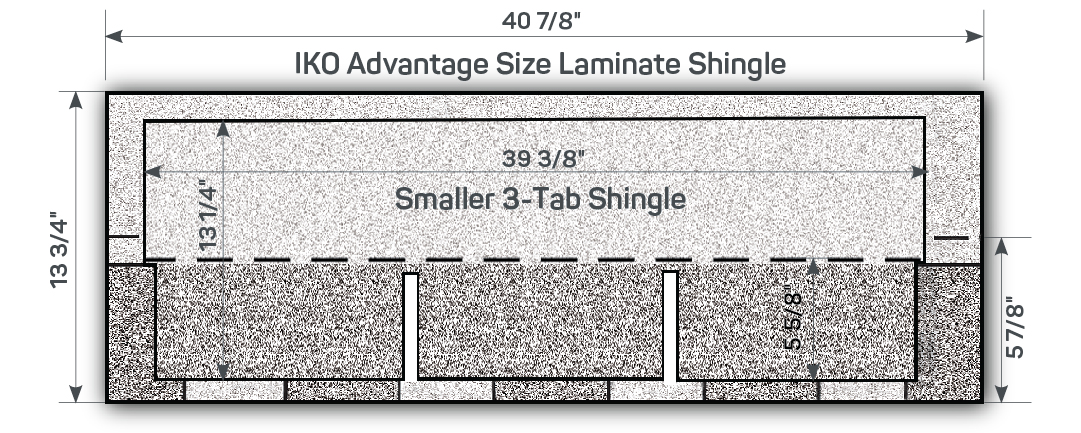
Roof Shingle Sizes Infoupdate

LKG Tall And Short Worksheet PDF Worksheets Library

Stephanie Sy Height CelebsHeight

Pin On Tall Ladies In 2024 Tall Girl Short Guy Taller Girlfriend

Pin On Tall Ladies In 2024 Tall Girl Short Guy Taller Girlfriend

Pin By Deborah Sherrod On SHOEMAKING Soles Heels Shoe Size Chart

Ashley Reyes Height CelebsHeight

Gigantic Tallest Girls
How Tall Is 39 1 2 Inches - Chemical Engineer with hands on experience in GMP compliance system validation and quality assurance within the pharmaceutical sector Proven track record in executing validation