What Is A Dilute Solution What happens when dilute hydrochloric acid is added to lead nitrate solution Q 100 m L of 0 01 M solution of N a O H is diluted to 1 d m 3 What is the p H of the diluted solution
Dilution is the process of reducing the concentration of a solute in solution usually simply by mixing with more solvent Example 1 You can add water to concentrated orange juice to dilute it until it reaches a concentration that is pleasant to drink Tap water is an example of a dilute solution it contains very small quantities of dissolved The dilute solution still has 10 grams of salt To prepare a fixed amount of dilute solution we have a formula C1V1 C2V2 Where V1 denotes the Volume of stock solution needed to make the new solution V2 is the final volume of the solution C1 Concentration of stock solution C2 Final concentration of stock solution Solved Examples
What Is A Dilute Solution

What Is A Dilute Solution
https://i.ytimg.com/vi/UoeKOvrzuzs/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLDH1okbXdtLVNygJBKjWQJKsao2wQ

Q 3 Neetu Has Two Test Tubes Containing Dilute Hydrochloric Acid And
https://i.ytimg.com/vi/Se0G3FL2SEA/maxresdefault.jpg
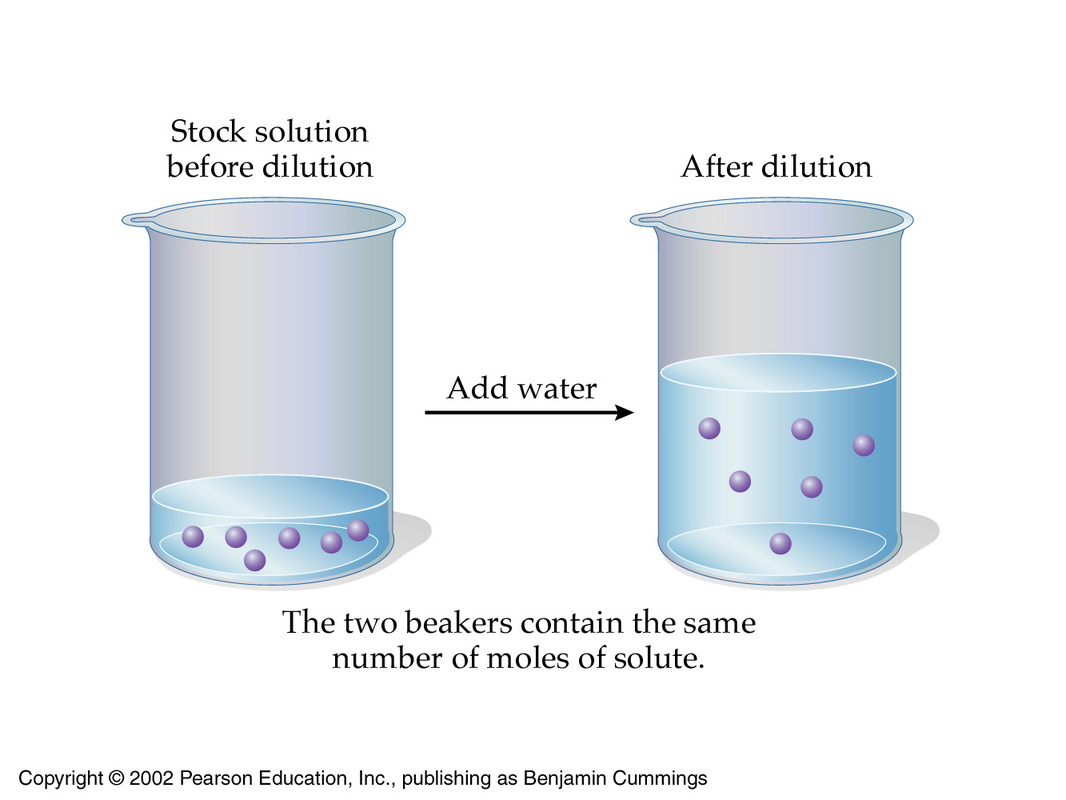
Molarity Acid And Bases For Dummies
http://acidsandbasesfordummieschem.weebly.com/uploads/2/7/8/0/27808149/6243113_orig.jpg
A saturated solution can be defined as a solution in which a solvent is not capable of dissolving any more solute at a given temperature The solutions are of two forms depending on whether the solvent is water or not Aqueous solution When a solute is dissolved in water the solution is called an aqueous solution Eg salt in water sugar Ideal dilute solution An ideal dilute solution is a true solution that obeys Henry s law and Raoult s law The interactions A A and B B have almost the same interactions due to which Ideal solutions are formed In pure ideal solutions the interactions will be of the A A and B B type and these interactions are due to the ideal behavior of
The solution in which the amount of solute is relatively more when compared to the amount of solvent is called concentrated solution It has less solute present in it It has a lot of solute present in it It can dissolve a lot more solute It can dissolve a little more solute only Example dilute sulphuric acid H 2 SO 4 dil dilute nitric Download Class 10 Chemistry Viva questions on the topic To find the pH of the dilute hydrochloric acid dilute NaOH solution dilute ethanoic acid solution lemon juice water and dilute sodium hydrogen carbonate solution samples using a pH paper or a universal indicator by clicking the button below Download PDF Read Also pH of Samples
More picture related to What Is A Dilute Solution

How Can A Solution Be Dilute Or Concentrated Brainly in
https://hi-static.z-dn.net/files/d40/d6146f710de87081f46d9b538b8916a7.jpg

Dilute Definition
https://i.ytimg.com/vi/VzhHK-0o3X8/maxresdefault.jpg
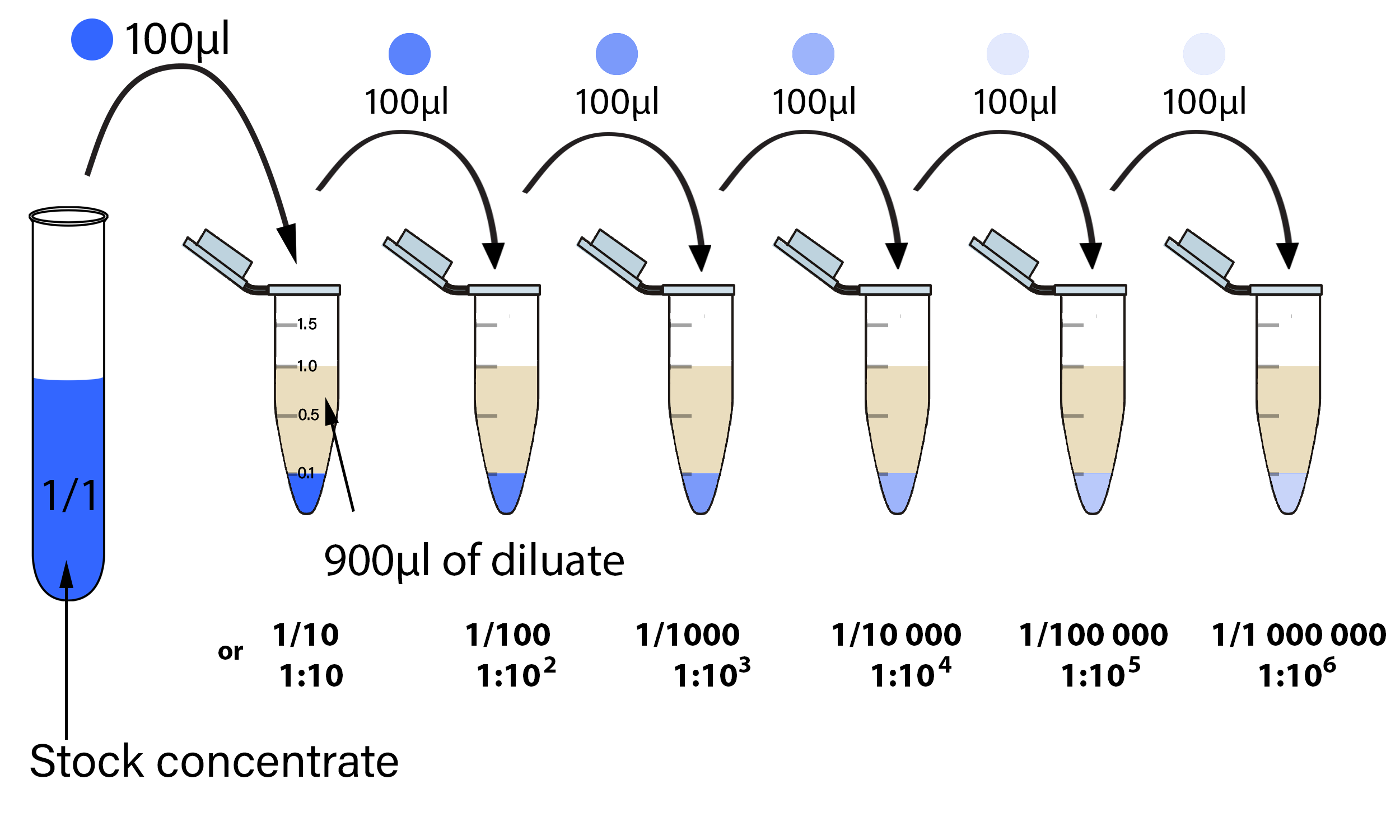
Serial Dilutions
http://www.medicine.mcgill.ca/physio/vlab/Vlab_in_progress/pics/Immunology/Serial_dilution.png
Supersaturated solution contains more dissolved substances than a saturated solution For example 40g NaCl in 100ml H 2 O The additional 4 0g NaCl remains undissolved Solved Examples 1 What is the mass percent of sodium hydroxide in a solution that is made by dissolving 8 00g NaOH in 50 0g H 2 O Solution Knowns 8 00g NaOH 50 0g H 2 O In a dilute solution N n Hence XB n N w m W M Where w and W are the weights of solute and solvent and m and M are their molecular weights respectively Therefore PoA PA PoA P PoA wM Wm Recommended Video Raoult s Law Ideal and Non Ideal Solutions
[desc-10] [desc-11]

Molarity
https://osmose-it.s3.amazonaws.com/71MsPnZETyaBVm7oZwrpn4lESEqd33yg/_.jpg

Quimica Para CENS 53 De La CABA Soluciones Concentracion De Una
https://1.bp.blogspot.com/-hn5AcZjVM0k/X0-_3gOubHI/AAAAAAAAVZw/aC4nD6yJ39UBFOozUpMWtyf1LgpJmM71QCNcBGAsYHQ/s1600/concentrated-and-dilute-solutions.jpg

https://byjus.com › question-answer › what-is-dilute-solution
What happens when dilute hydrochloric acid is added to lead nitrate solution Q 100 m L of 0 01 M solution of N a O H is diluted to 1 d m 3 What is the p H of the diluted solution

https://byjus.com › question-answer › definition-of-dilute-solution
Dilution is the process of reducing the concentration of a solute in solution usually simply by mixing with more solvent Example 1 You can add water to concentrated orange juice to dilute it until it reaches a concentration that is pleasant to drink Tap water is an example of a dilute solution it contains very small quantities of dissolved

Question Video Calculating The Volume Of Water Added To Dilute A

Molarity

Dilute Acid

Dilute Acid

What Is The Difference Between A Dilute Solution And A Concentrated

Lesson 8 The Chemistry Of Solutions

Lesson 8 The Chemistry Of Solutions
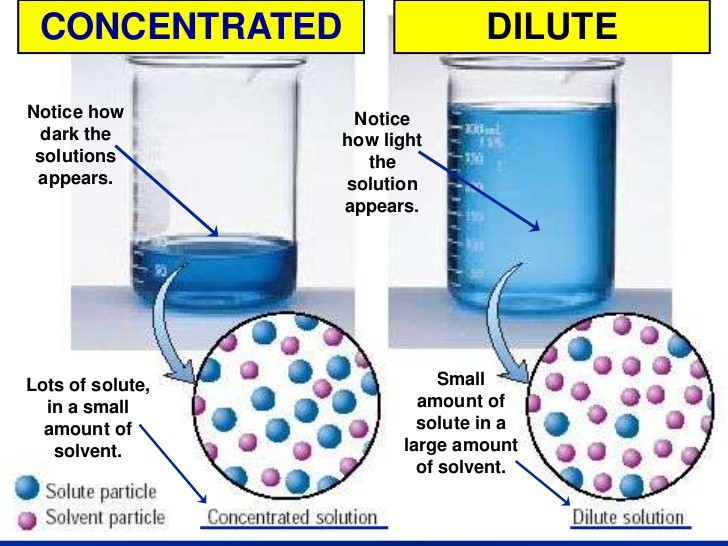
Types Of Solutions Study Material For IIT JEE AskIITians

Acid Metal Reaction

How To Dilute Solutions 8 Steps with Pictures WikiHow
What Is A Dilute Solution - [desc-12]