What Is A Diprotic Acid A diprotic acid is an acid that can donate two hydrogen ions H per molecule during a chemical reaction This means that each molecule of a diprotic acid has two acidic hydrogen atoms
Diprotic acids such as sulfuric acid H 2 SO 4 carbonic acid H 2 CO 3 hydrogen sulfide H 2 S chromic acid H 2 CrO 4 and oxalic acid H 2 C 2 O 4 have two acidic hydrogen atoms Definition A diprotic acid is an acid that can donate two proton or hydrogen atom per molecule to an aqueous solution Compare this to a monoprotic acid
What Is A Diprotic Acid

What Is A Diprotic Acid
https://slideplayer.com/slide/16660476/96/images/8/Monoprotic+vs.+diprotic+vs.+triprotic+acids.jpg

Strong And Weak Acids A Level ChemistryStudent
https://www.chemistrystudent.com/images/A2Physical/acidsandbases/strongandweakacids3.png
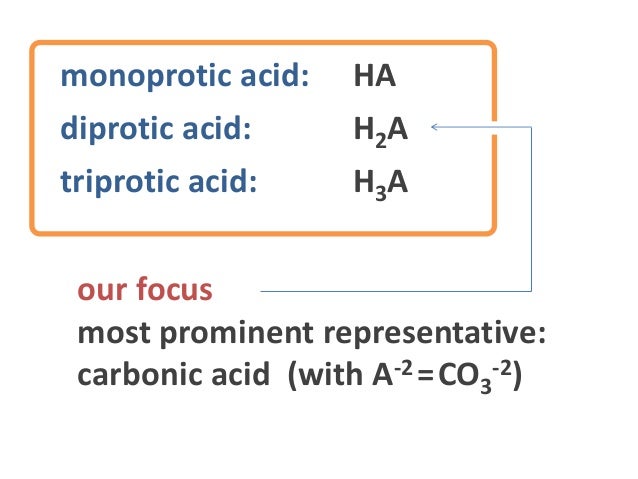
Diprotic Acids And Equivalence Points
https://image.slidesharecdn.com/diproticacid3-160528081306/95/diprotic-acids-and-equivalence-points-2-638.jpg?cb=1492249082
In chemistry a diprotic acid is an acid that can donate two hydrogen atoms H or protons per each molecule of the acid to a solution that is in an aqueous state or in water A diprotic acid is a type of acid that has the capacity to donate two protons or hydrogen ions H per molecule in an aqueous solution This donation of protons occurs in a stepwise manner
A diprotic acid is an acid that has two hydrogen ions in its molecule Diprotic acids are capable of donating a total of two hydrogen ions to other molecules Sulfuric acid has two so it would be called a di protic acid Phosphoric acid has three so it s called a tri protic acid EDTA has four acidic protons meaning it would technically be called a
More picture related to What Is A Diprotic Acid

Is Dibasic Acids Same As Diprotic Acids
http://www.simplechemconcepts.com/wp-content/uploads/2014/02/O-Level-Chemistry-Tuition_Basicity-of-Acids.jpg

Weak Diprotic Acid Titration Curve Godwin Aces1963
https://www.chemistryguru.com.sg/images/diprotic_acid_titration_curve_-_sketch_titration_curve.png
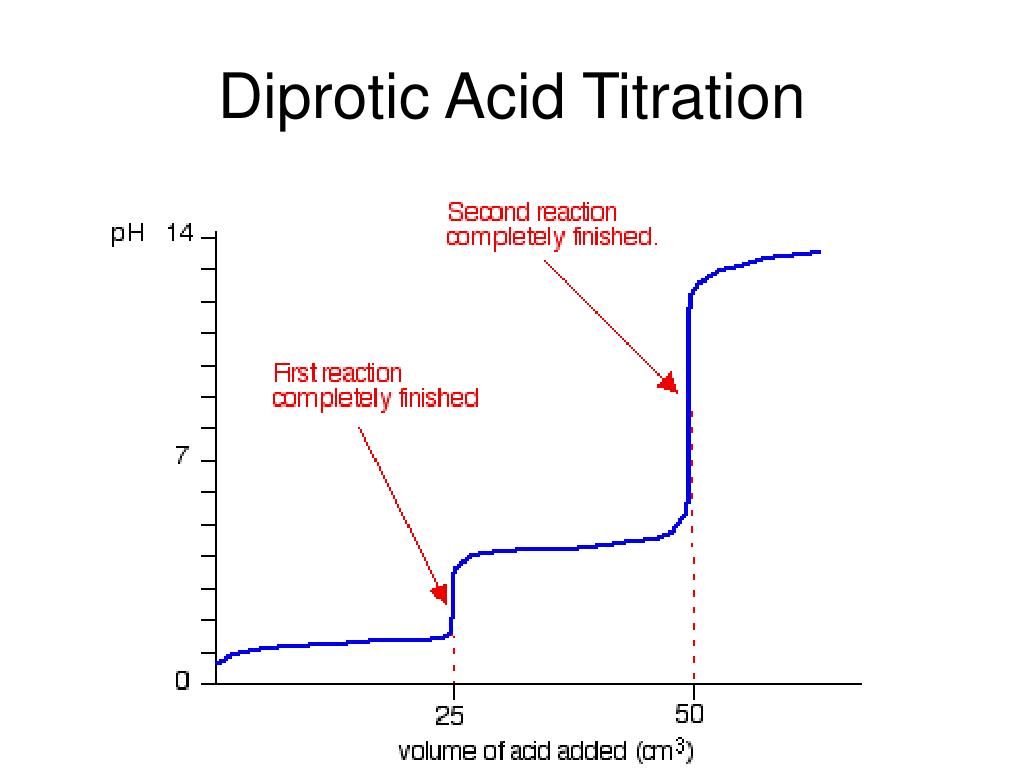
Diprotic Acid Titration Curve
https://image3.slideserve.com/5723241/diprotic-acid-titration-l.jpg
A diprotic acid is an acid that can donate two protons H per molecule in a solution This characteristic allows diprotic acids to undergo two distinct dissociation steps each with its own As for the diprotic acid examples each successive ionization reaction is less extensive than the former reflected in decreasing values for the stepwise acid ionization
[desc-10] [desc-11]
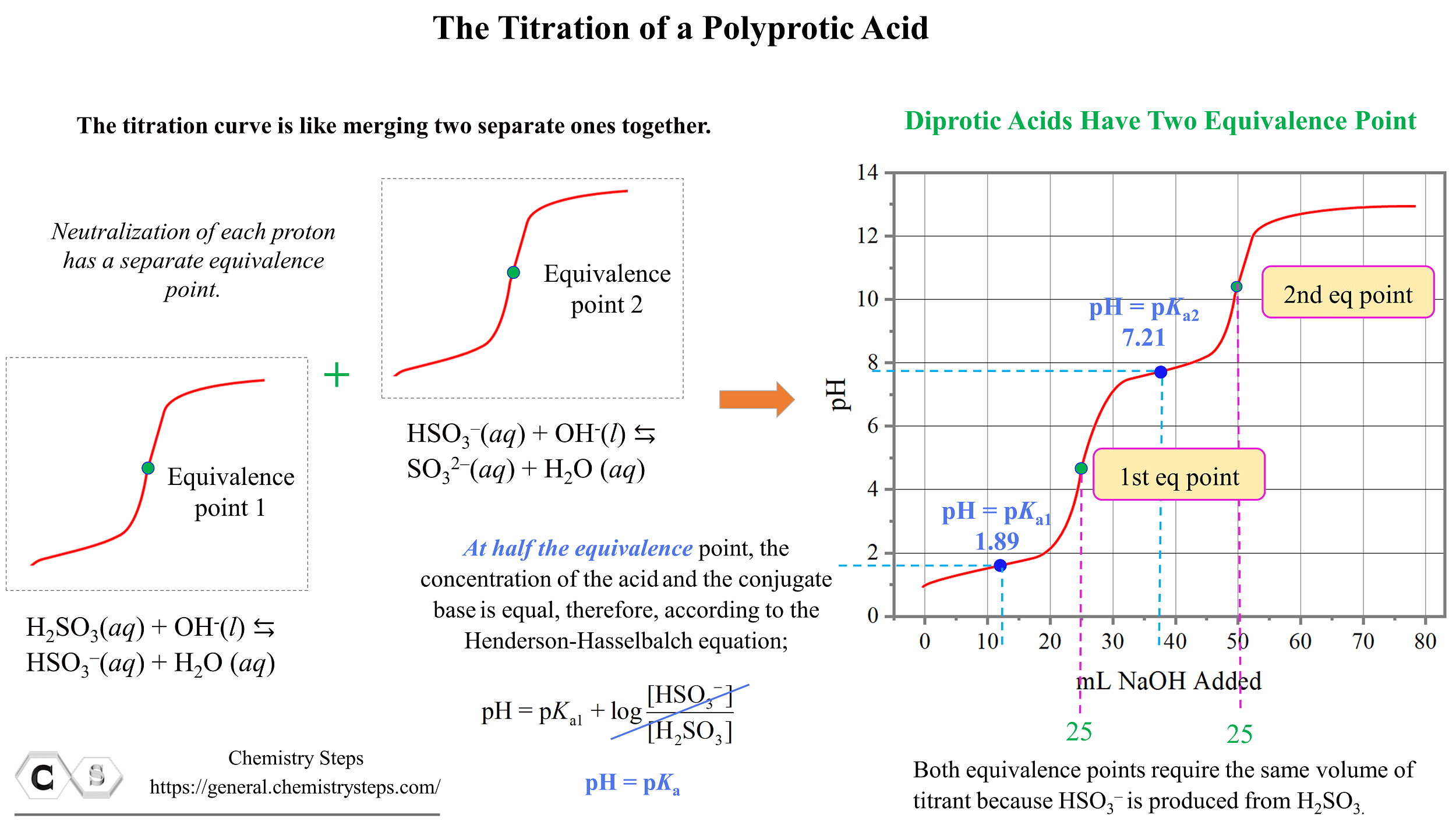
Titration Curve Diprotic Acid
https://general.chemistrysteps.com/wp-content/uploads/2022/07/The-titration-curve-for-Polyprotic-Acids.png
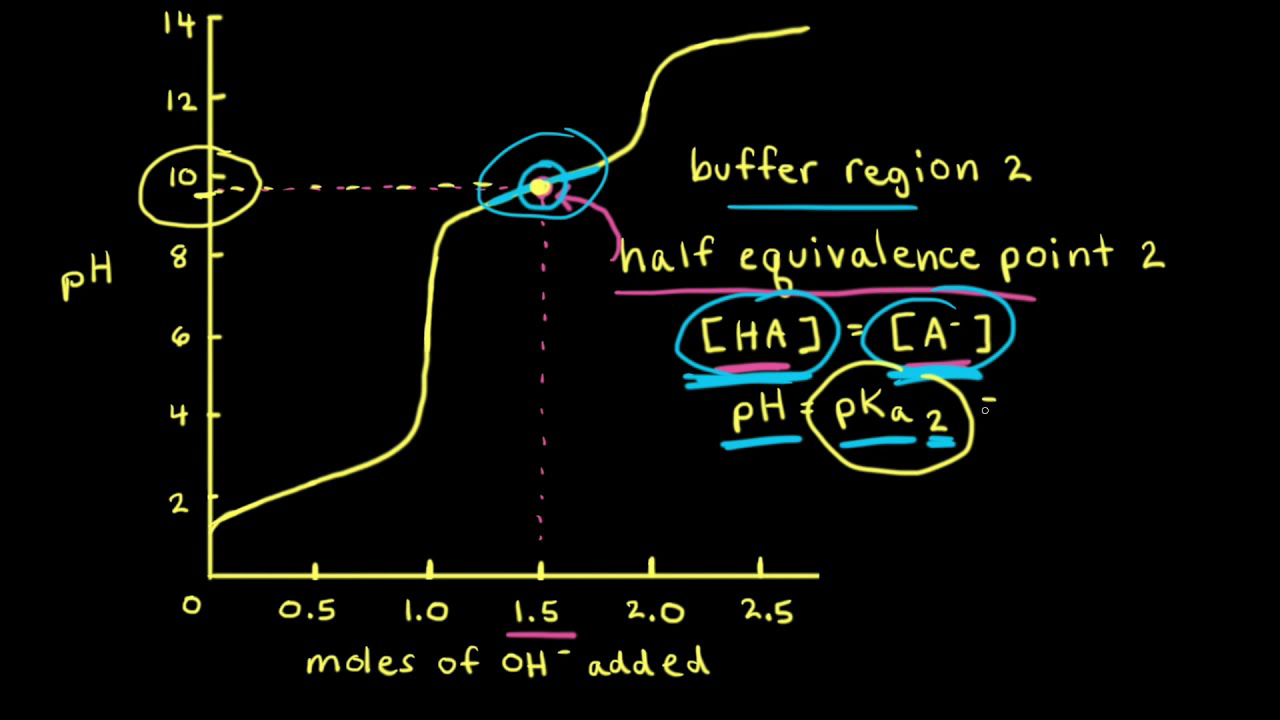
Titration Curve Diprotic Acid
https://cdn.kastatic.org/ka_thumbnails_cache/b3ce938a-a9d5-4778-8edd-d40f8eb55237_1280_720_base.png

https://thisvsthat.io › diprotic-acid-vs-monoprotic-acid
A diprotic acid is an acid that can donate two hydrogen ions H per molecule during a chemical reaction This means that each molecule of a diprotic acid has two acidic hydrogen atoms

https://chemed.chem.purdue.edu › ... › diprotic.php
Diprotic acids such as sulfuric acid H 2 SO 4 carbonic acid H 2 CO 3 hydrogen sulfide H 2 S chromic acid H 2 CrO 4 and oxalic acid H 2 C 2 O 4 have two acidic hydrogen atoms

Titration Curve Diprotic Acid

Titration Curve Diprotic Acid

Titration Curve Diprotic Acid

Titration Curve Diprotic Acid

Titration Curve Diprotic Acid
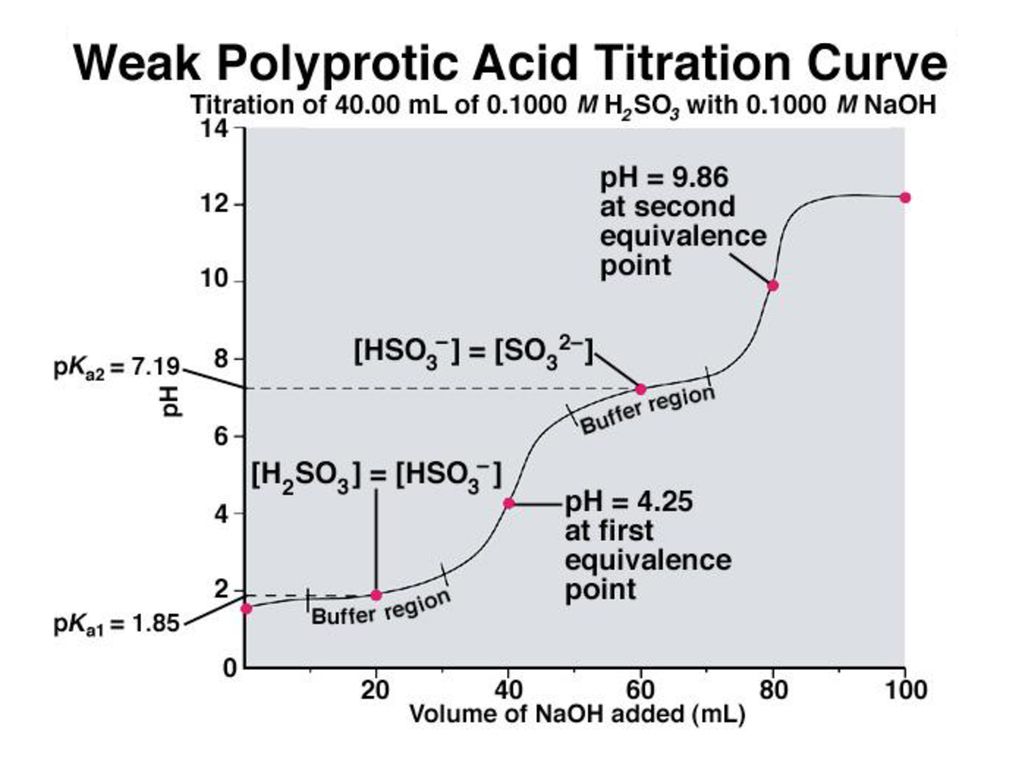
Titration Curve Diprotic Acid

Titration Curve Diprotic Acid
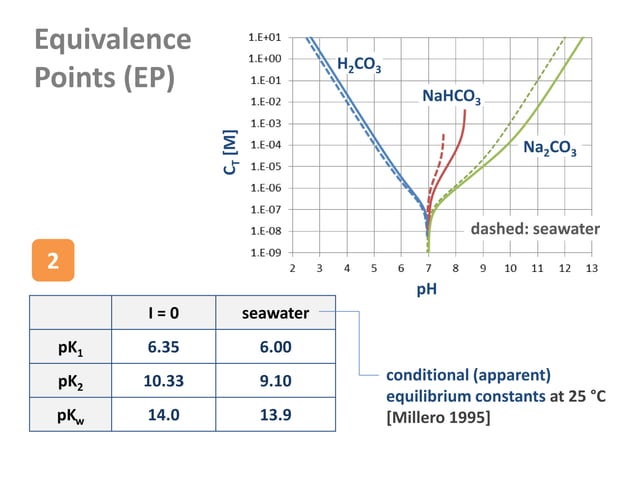
Diprotic Acids And Equivalence Points PPT

11

11
What Is A Diprotic Acid - A diprotic acid is a type of acid that has the capacity to donate two protons or hydrogen ions H per molecule in an aqueous solution This donation of protons occurs in a stepwise manner