What Is Dilution Of Acid And Base Dilution is the addition of solvent which decreases the concentration of the solute in the solution Concentration is the removal of solvent which increases the concentration of the solute in the
Dilution is a fundamental concept in chemistry that involves reducing the concentration of a substance in a solution by adding a solvent It is a technique that is used to create less Dilution is the process of decreasing the concentration of a solute in a solution usually simply by mixing with more solvent like adding more water to the solution
What Is Dilution Of Acid And Base

What Is Dilution Of Acid And Base
https://i.ytimg.com/vi/CxEt3Ge7XXU/maxresdefault.jpg

What Is Dilute Solution Chemistry YouTube
https://i.ytimg.com/vi/eVhJnFpRqG0/maxresdefault.jpg

CHEMISTRY 101 Solution Dilutions YouTube
https://i.ytimg.com/vi/E5GDC_q_Wus/maxresdefault.jpg
The dilution is by a factor of 32 to go from 16 M to 0 5 M Image 1 Volumetric pipette Image from the Teaching and Learning Centre Figure 2 Micropipette Image by rocksee Dilutions can be The act of reducing the concentration of a solution by adding additional solvent is known as dilution Dilution involves adding additional solvent to a sample of a solution The process does
This process is known as dilution because relative to the solution from which it was prepared the final solution contains the same amount of solute in a larger volume of solution and therefore Definition Dilution is the act of adding a solvent usually water to a solution What is dilution Dilution is when you add a solvent to a solution In A level Chemistry dilution only occurs with
More picture related to What Is Dilution Of Acid And Base
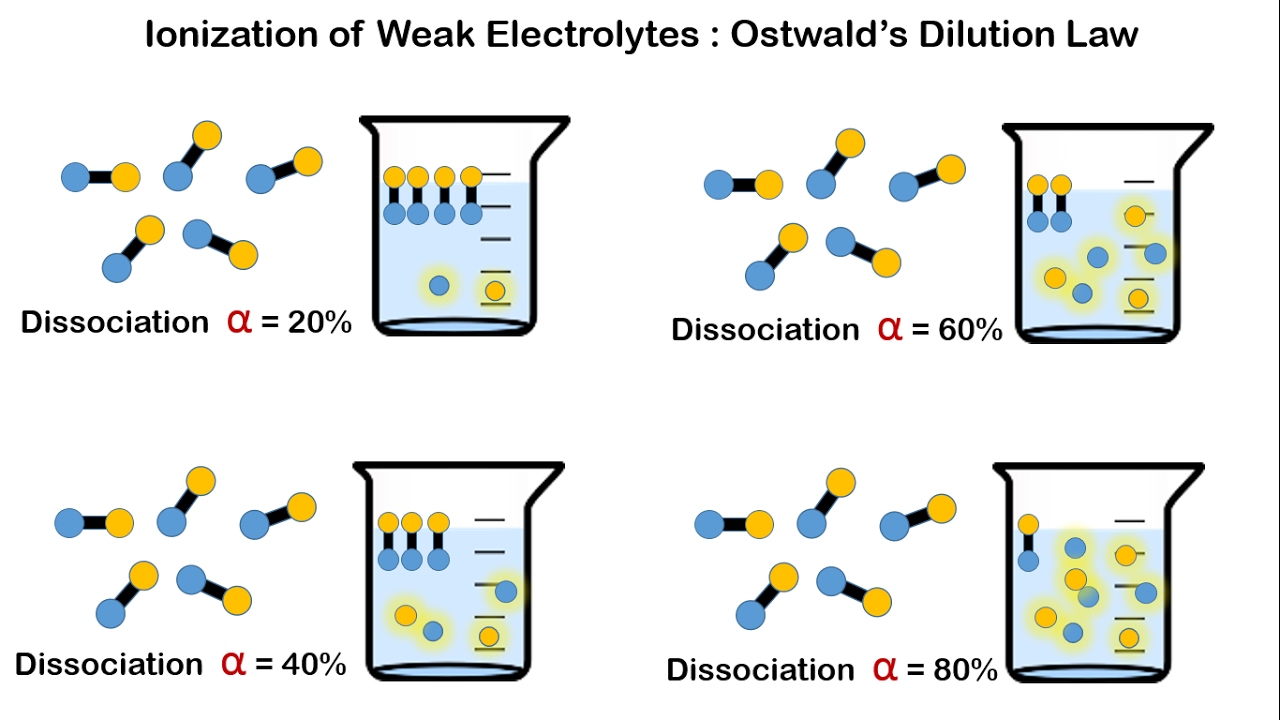
Ionisation Of Weak Electrolytes And Ostwalds Dilution Law YouTube
https://i.ytimg.com/vi/o4CxxRAPc0Y/maxresdefault.jpg

Dilution Lab Results Chemistry Matters YouTube
https://i.ytimg.com/vi/JJ6d8xctq7g/maxresdefault.jpg

Serial Dilutions And Plating Microbial Enumeration 46 OFF
https://iul-instruments.com/wp-content/uploads/2021/01/[email protected]
The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment analysis or application The process of dilution follows a simple mathematical The sub one refers to the situation before dilution and the sub two refers to after dilution This equation does not have an official name like Boyle s Law so we will just call it the dilution
[desc-10] [desc-11]

Dilution When Solvent Is Added To Dilute A Solution The Number Of
https://i.pinimg.com/originals/b5/f9/04/b5f904fc793680d474f6351e36ac239c.jpg

Derive Ostwald s Dilution Law For Weak Acid Ch3cooh Brainly in
https://hi-static.z-dn.net/files/d73/04772b987c9d03d18975d9a0f81e12f0.jpg

https://chem.libretexts.org › Bookshelves...
Dilution is the addition of solvent which decreases the concentration of the solute in the solution Concentration is the removal of solvent which increases the concentration of the solute in the

https://testbook.com › chemistry › dilute-definition
Dilution is a fundamental concept in chemistry that involves reducing the concentration of a substance in a solution by adding a solvent It is a technique that is used to create less
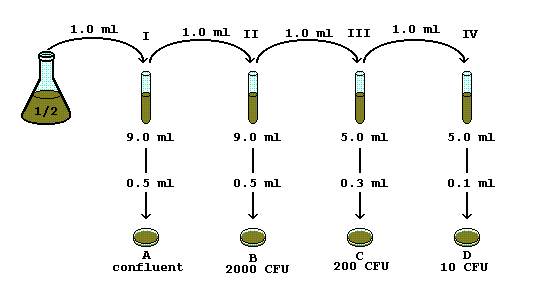
Serial Dilution Problem Help

Dilution When Solvent Is Added To Dilute A Solution The Number Of
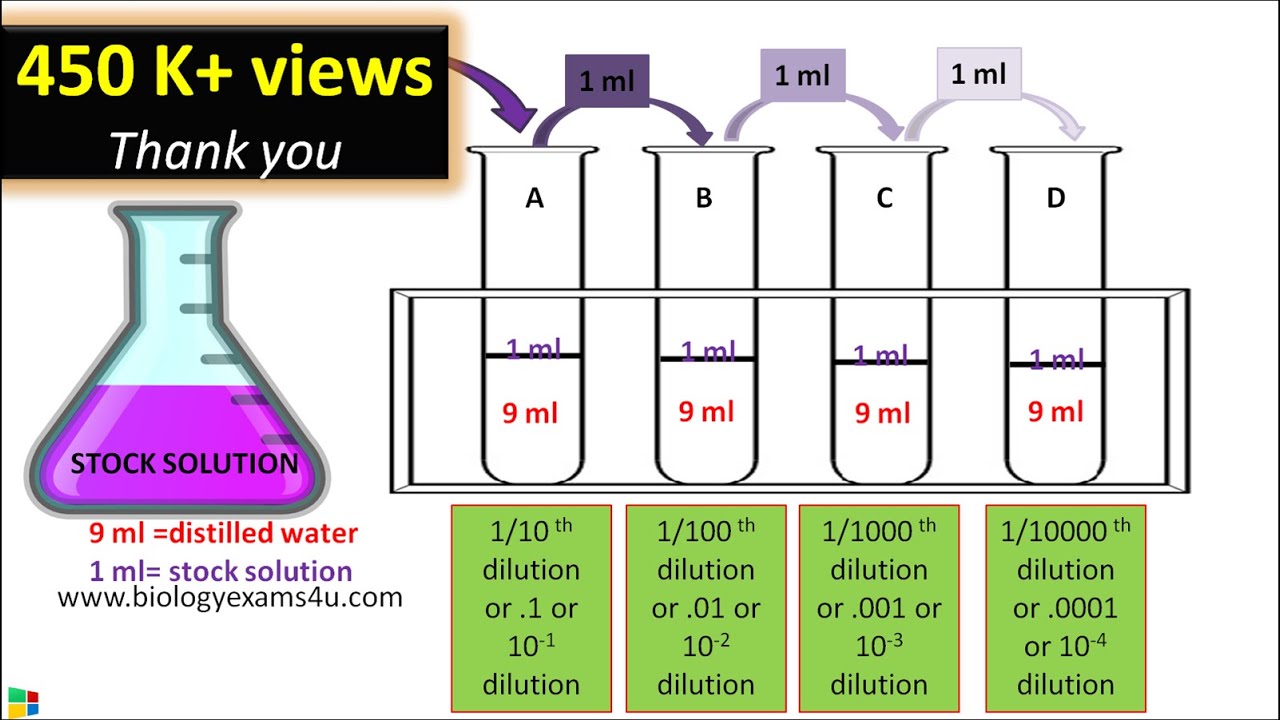
Serial Dilution Method Protocol Step Wise Explanation YouTube
Solutions Part 1 Solutions Preparation Used In Clinical Laboratory
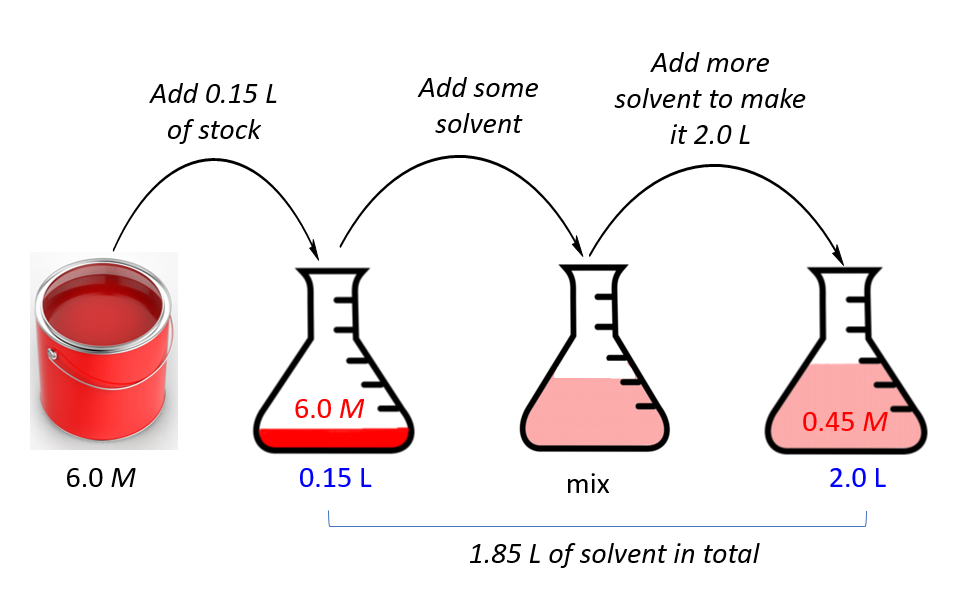
Dilution Of A Stock Solution And Calculations Based Morality

Calculate Dilution Factor Promolasopa

Calculate Dilution Factor Promolasopa

Overview Of Acid Base Regulation Osmosis Video Library

PH ACIDS AND ALKALIS

Chemical Dilution Rate Guide Dalcon Hygiene
What Is Dilution Of Acid And Base - [desc-14]