What Is Dilution Of Acid Class 10 Dilution is the addition of solvent which decreases the concentration of the solute in the solution Concentration is the removal of solvent which increases the concentration of
Dilution is the process of decreasing the concentration of a solute in a solution usually simply by mixing with more solvent like adding more water to the solution To dilute a solution means to DILUTION definition 1 the action of making a liquid weaker by mixing in something else or a liquid that has been made Learn more
What Is Dilution Of Acid Class 10

What Is Dilution Of Acid Class 10
https://i.ytimg.com/vi/CxEt3Ge7XXU/maxresdefault.jpg

How To Use The Dilution Equation YouTube
https://i.ytimg.com/vi/JTKnmOp0I8g/maxresdefault.jpg
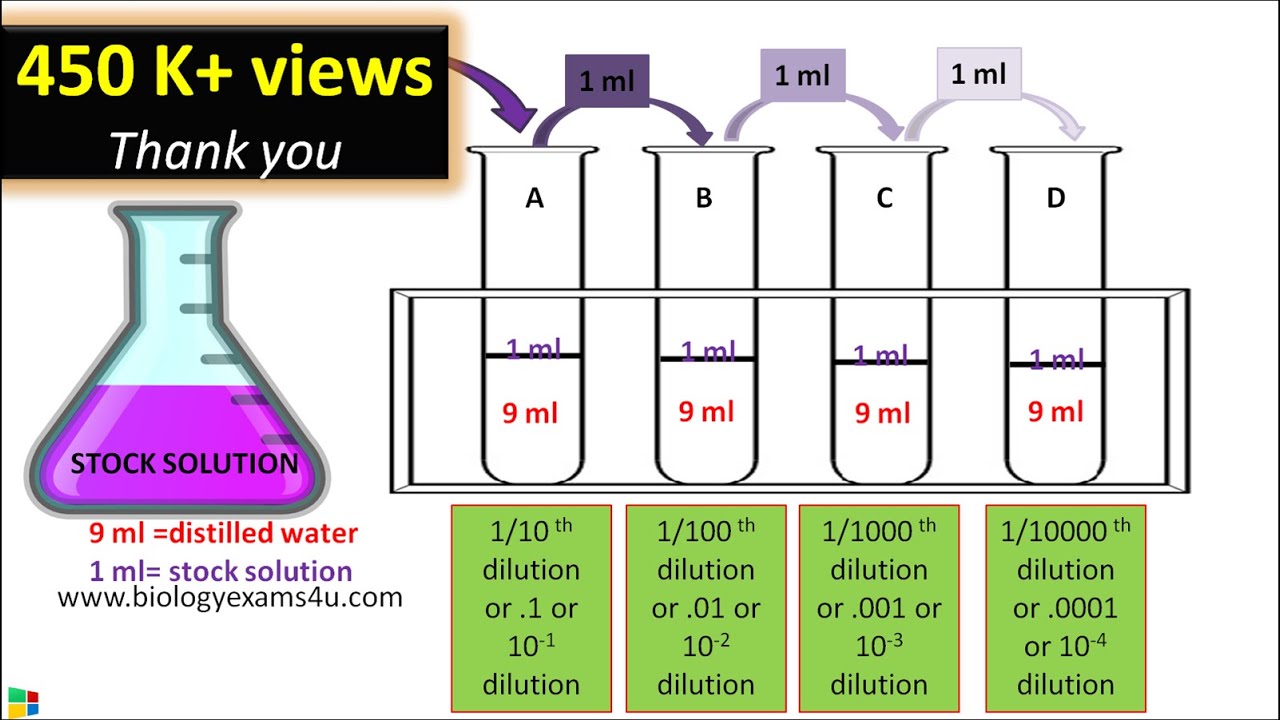
Serial Dilution Method Protocol Step Wise Explanation YouTube
https://i.ytimg.com/vi/MCrNjHcfcpY/maxresdefault.jpg
Dilution is a fundamental concept in chemistry that involves reducing the concentration of a substance in a solution by adding a solvent It is a technique that is used to create less Dilution is the addition of water to reduce concentration Dilution does not change the number of moles Dilution changes the E value of the atoms or ions during electrolysis
Another common dilution problem involves calculating what amount of a highly concentrated solution is required to make a desired quantity of solution of lesser concentration The highly Dilution is the process of making a solution or scientific sample less concentrated by adding more solvent For example if you have a glass of lemonade but it is too sour you might add more
More picture related to What Is Dilution Of Acid Class 10

What Is Dilution Chemistry Matters YouTube
https://i.ytimg.com/vi/lMTaNO5w4zg/maxresdefault.jpg

Properties Of Acid Class 10 YouTube
https://i.ytimg.com/vi/ymNtnYlfgig/maxres2.jpg?sqp=-oaymwEoCIAKENAF8quKqQMcGADwAQH4Ac4FgAKACooCDAgAEAEYZSBlKGUwDw==&rs=AOn4CLC5TOGGmLb6wmgiflXOQQBRlPsTJw

Properties Of Acid Basicity Of Acid Class 10 Chemistry Class 12
https://i.ytimg.com/vi/4xwpRebHrlw/maxresdefault.jpg
Using the dilution equation we write 1 50 mol L 53 4 mL 0 800 mol L x x 100 mL Notice that the volumes need not be converted to liters Any old volume measurement is fine Dilution is the act of lowering the concentration of a solute in a solution by mixing it with various solvents such as water Adding extra solvent to a solution without adding more solute is
[desc-10] [desc-11]

CHEMISTRY 101 Solution Dilutions YouTube
https://i.ytimg.com/vi/E5GDC_q_Wus/maxresdefault.jpg
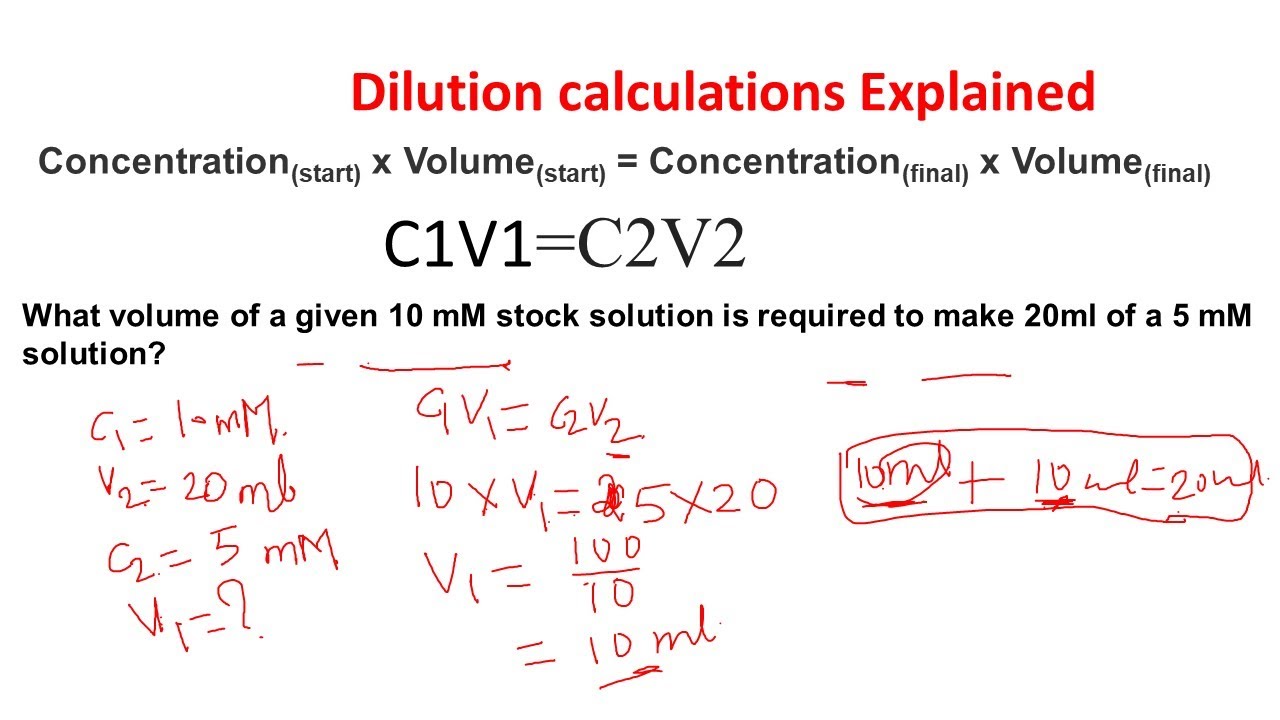
Dilution Calculations Dilution Problems Stock Dilutions Biology And
https://i.ytimg.com/vi/oNBdTnHJY1A/maxresdefault.jpg

https://chem.libretexts.org › Bookshelves › Introductory_Chemistry...
Dilution is the addition of solvent which decreases the concentration of the solute in the solution Concentration is the removal of solvent which increases the concentration of

https://en.wikipedia.org › wiki › Dilution_(equation)
Dilution is the process of decreasing the concentration of a solute in a solution usually simply by mixing with more solvent like adding more water to the solution To dilute a solution means to

Chemical Properties Of Acid Class 10 YouTube

CHEMISTRY 101 Solution Dilutions YouTube

Dilution Lab Results Chemistry Matters YouTube

Dilution And Dilution Factor In Microbiology How To Calculate

Serial Dilutions And Plating Microbial Enumeration 46 OFF
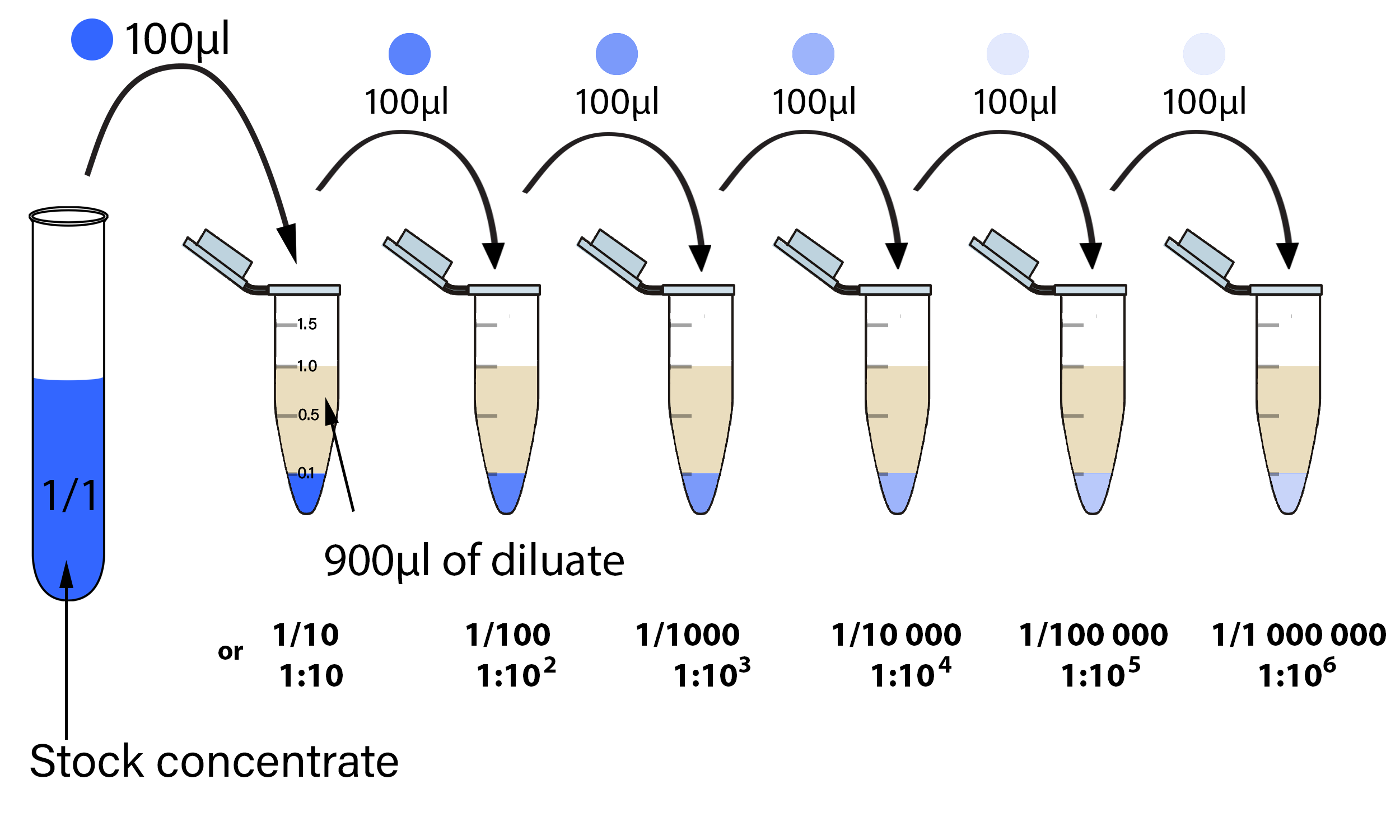
Serial Dilutions And Plating Microbial Enumeration 46 OFF

Serial Dilutions And Plating Microbial Enumeration 46 OFF

Serial Dilution
Solutions Part 1 Solutions Preparation Used In Clinical Laboratory
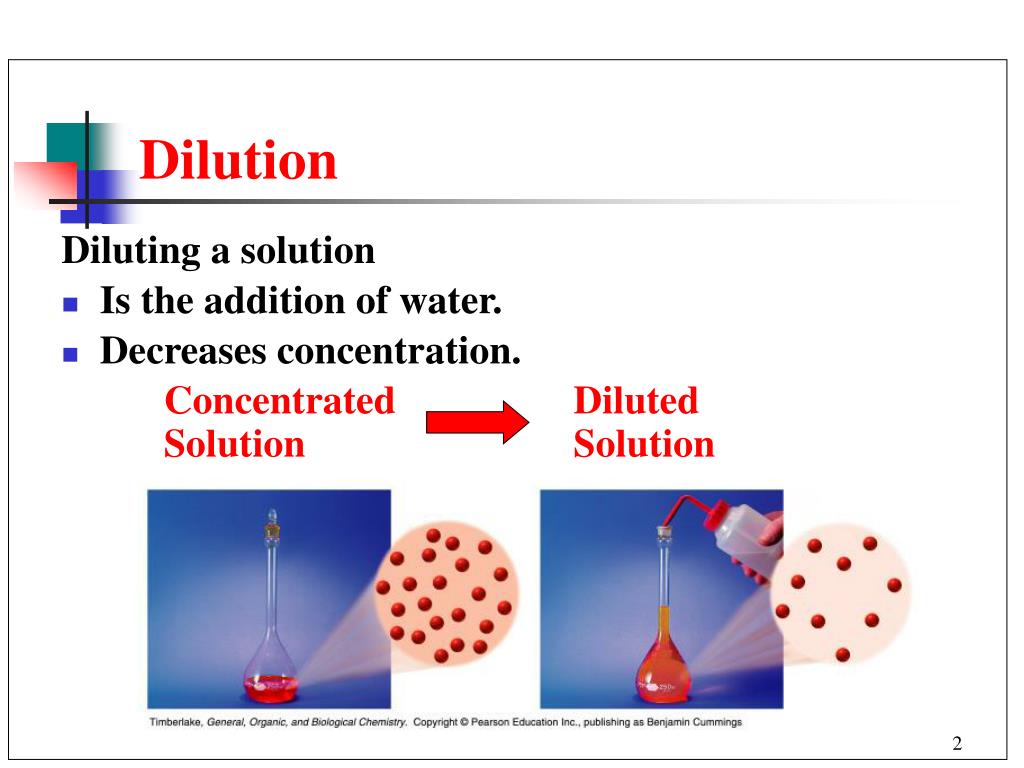
Regression Dilution Wikipedia
What Is Dilution Of Acid Class 10 - Another common dilution problem involves calculating what amount of a highly concentrated solution is required to make a desired quantity of solution of lesser concentration The highly