What To Know About Sinovacs Corona Virus Vaccine Coronavac This quick guide offers basic information about COVID 19 the Sinovac CoronaVac COVID 19 vaccine and what to expect following vaccination 1 What is COVID 19 2 Who is most at risk
As of 7 July 2021 CoronaVac is the most widely used COVID 19 vaccine in the world with 943 million doses delivered globally In July Sinovac signed advanced purchase agreements with GAVI to supply COVAX with 50 million doses in the third quarter of 2021 and up to a total of 380 million doses by the first half of 2022 Starting from 1 June 2021 the World Health Organisation WHO has approved the use of the Sinovac CoronaVac COVID 19 vaccine under its Emergency Use Listing EUL This means that Sinovac vaccine has been assessed to meet
What To Know About Sinovacs Corona Virus Vaccine Coronavac
What To Know About Sinovacs Corona Virus Vaccine Coronavac
https://yalibnan.com/wp-content/uploads/2020/10/COVID-19-Coronavirus-Vaccine-1024x682.jpg
![]()
Coronavirus Variant Tracker The New York Times
https://static01.nyt.com/newsgraphics/2021/01/22/coronavirus-variant-tracker/223ad990cf28c45e5a03241de8c5a677bd3ac7b3/coronavirus-intro-900.png
Coronavirus Vaccine Treatments Cure Sought Like For SARS MERS
https://www.gannett-cdn.com/-mm-/ff08101970c9b392424972bf0f5861a9adca4d8f/c=0-110-2122-1304/local/-/media/2020/03/18/USATODAY/usatsports/MotleyFool-TMOT-8bf000a1-coronavirus-vaccine.jpg?width=2122&height=1194&fit=crop&format=pjpg&auto=webp
Sinovac s coronavirus disease COVID 19 vaccine is known as SARS CoV 2 vaccine Vero Cell Inactivated or the CoronaVac CoronaVac is the third vaccine to be granted The Sinovac COVID 19 vaccine is available in Singapore for use against the COVID 19 virus Read on to find out more about how the vaccine works its efficacy safety and how it compares with the Pfizer BioNTech and
CoronaVac also known as the Sinovac inactivated SARS CoV 2 vaccine has been widely implemented in combating the COVID 19 pandemic We summarized the results of clinical trials and real world studies of CoronaVac in this review Sinovac CoronaVac compared to unvaccinated individuals showed the following findings the adjusted vaccine effectiveness against symptomatic COVID 19 was 79 95
More picture related to What To Know About Sinovacs Corona Virus Vaccine Coronavac
Share Your Coronavirus And Vaccine Stories
https://www.gannett-cdn.com/media/2021/02/10/USATODAY/usatsports/covid-19-vaccine-getty.jpg?crop=2245,1263,x0,y36&width=2245&height=1263&format=pjpg&auto=webp

Did You Get The Coronavirus Vaccine Or Are You Planning To The New
https://static01.nyt.com/images/2020/12/14/us/covid-vaccine-callout-1/merlin_181253976_f7916360-8e5e-4bc0-9101-a5488f9370a2-videoSixteenByNine3000.jpg
A Potencial Vantagem Da CoronaVac Contra Variantes Do Coronav rus BBC
https://ichef.bbci.co.uk/news/1024/branded_portuguese/1A29/production/_116879660_coronavac.jpg
Sinovac CoronaVac is an aluminium hydroxide adjuvanted inactivated whole virus vaccine which was granted WHO Emergency Use Listing EUL in May 2021 The WHO EUL The results indicate that receiving CoronaVac vaccine as a booster dose is effective in preventing SARS CoV 2 Omicron BA 2 infection in the short term However the efficacy of CoronaVac
The Sinovac vaccine CoronaVac is an inactivated vaccine developed by a private Chinese company It requires two doses given 2 4 weeks apart similar to mRNA vaccines and Starting from 1 June 2021 the World Health Organisation WHO has approved the use of the Sinovac CoronaVac COVID 19 vaccine under its Emergency Use Listing EUL This means

Coronavirus At Least 50 Priests Killed By Coronavirus BBC News
https://ichef.bbci.co.uk/news/240/cpsprodpb/D502/production/_111403545_optimised-coronavirus_global_area_chart_jh_24mar-nc.png
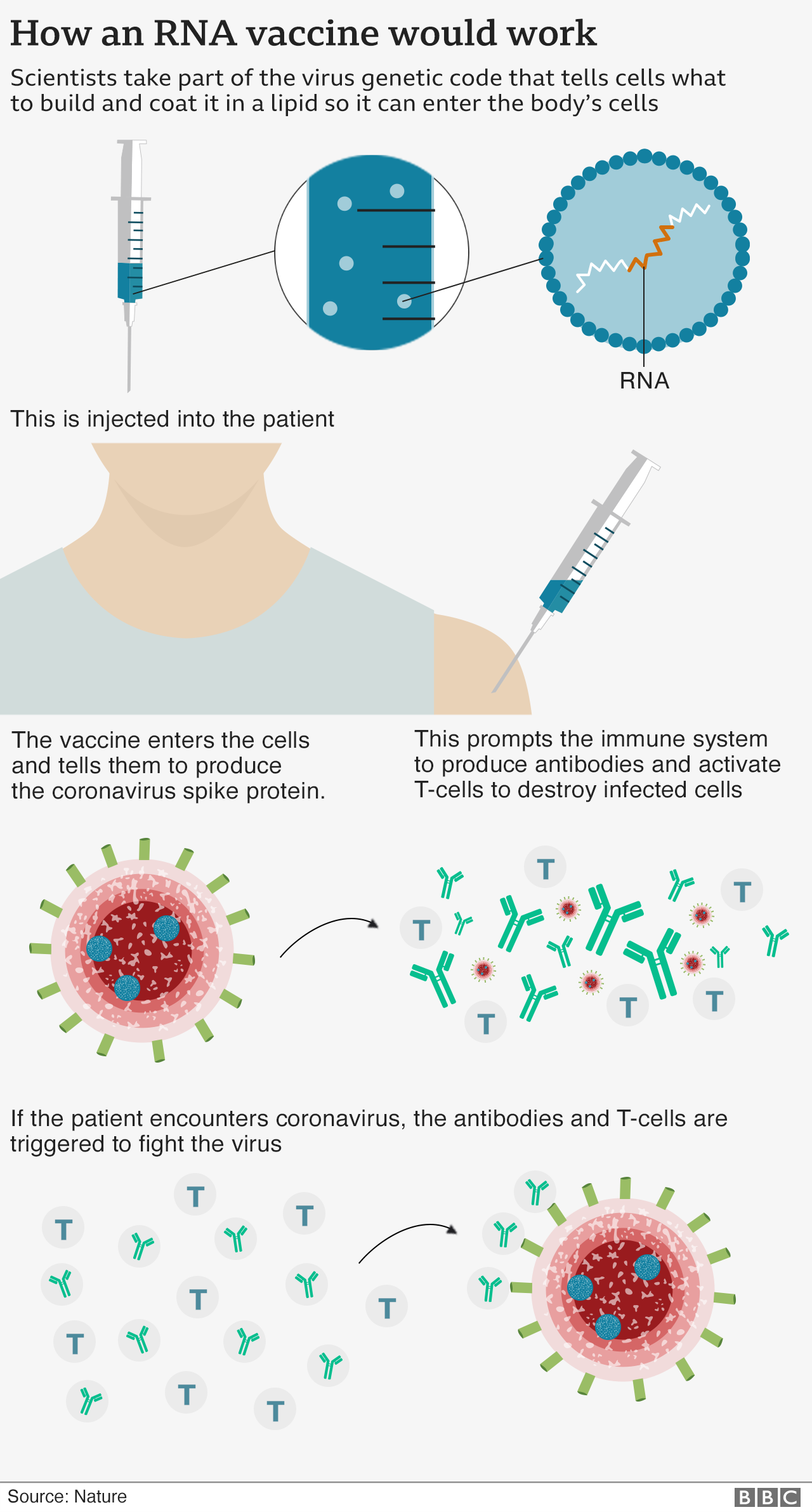
Virus Corona Cu i C ng Ch ng Ta C Thu c Ch ng Ng a Covid BBC
https://ichef.bbci.co.uk/news/640/cpsprodpb/648E/production/_115324752_covid19_how_vaccines_work2_v3-nc.png
https://www.who.int › ... › default-document-library
This quick guide offers basic information about COVID 19 the Sinovac CoronaVac COVID 19 vaccine and what to expect following vaccination 1 What is COVID 19 2 Who is most at risk
https://en.wikipedia.org › wiki › CoronaVac
As of 7 July 2021 CoronaVac is the most widely used COVID 19 vaccine in the world with 943 million doses delivered globally In July Sinovac signed advanced purchase agreements with GAVI to supply COVAX with 50 million doses in the third quarter of 2021 and up to a total of 380 million doses by the first half of 2022

3 Potential Coronavirus Vaccines Showing Promise In Early Tests Fox News

Coronavirus At Least 50 Priests Killed By Coronavirus BBC News
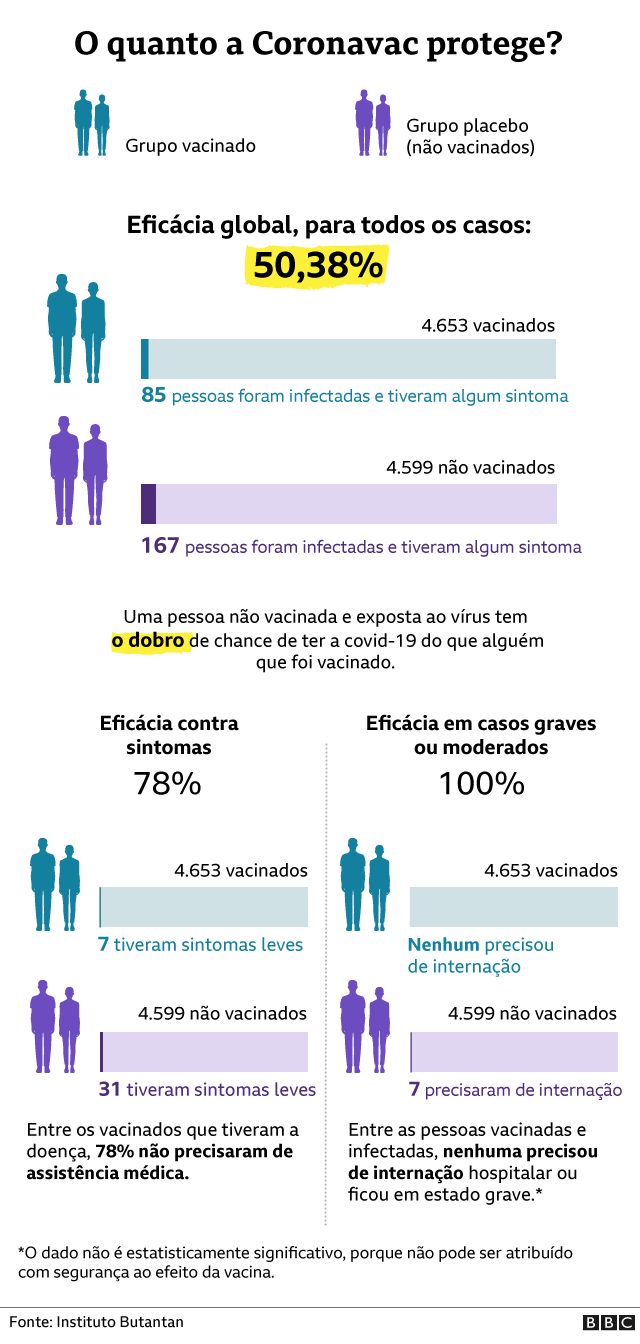
Coronav rus A Efic cia Da CoronaVac E Demais Vacinas Explicada Em 4
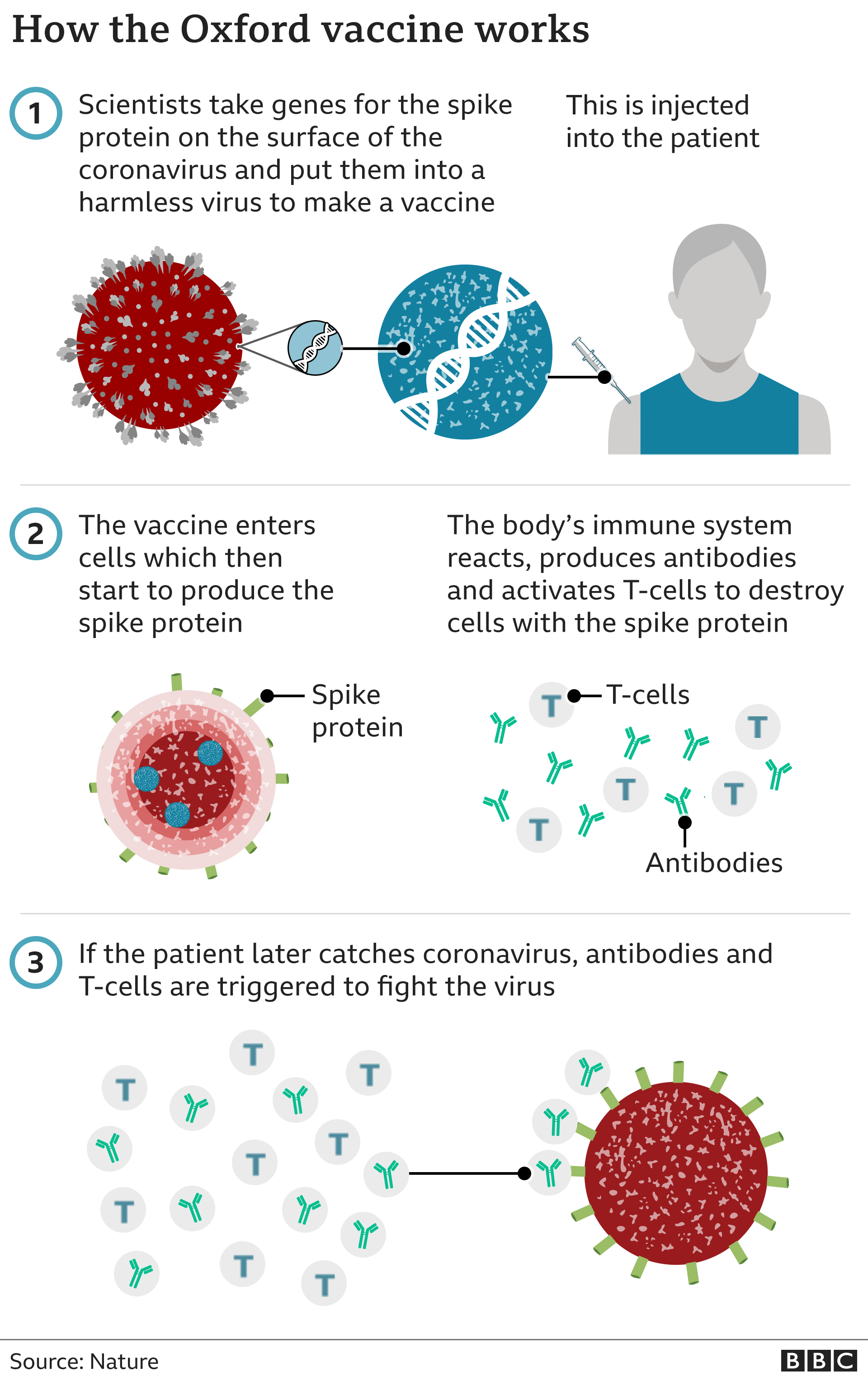
Covid How Does The Oxford AstraZeneca Vaccine Work BBC News
COVID 19 Vaccine

Brazil Coronavirus Vaccine Sinovac s CoronaVac Trial Results

Brazil Coronavirus Vaccine Sinovac s CoronaVac Trial Results
Experimental Coronavirus Vaccine Is Safe And Produces Immune Response
Experimental Coronavirus Vaccine Highly Effective National Institutes
Coronavirus Trust In Vaccines Could Turn On A Knife Edge Bloomberg
What To Know About Sinovacs Corona Virus Vaccine Coronavac - The Sinovac vaccine is a vaccine that uses an inactivated form of the COVID 19 virus instead of the mRNA technology that Pfizer and Moderna use Developed by Chinese biopharmaceutical