2x 5 12 8 Methane CH burns in the presence of oxygen O to produce carbon dioxide CO and water H O according to the following balanced chemical equation CH 2O CO 2H O
Answer When methane CH4 burns it reacts with oxygen gas to produce carbon dioxide and water The unbalanced equation for this reaction is CH4 g O2 g When methane CH is burned in an insufficient supply of oxygen the combustion reaction does not proceed completely leading to the formation of carbon monoxide CO instead of carbon
2x 5 12 8

2x 5 12 8
https://i.ytimg.com/vi/-VFx7xdSR3I/maxresdefault.jpg

Expand Simplify 2x 3 3x 4 YouTube
https://i.ytimg.com/vi/y0HY15Dp-5g/maxresdefault.jpg

Solve 2x 3y 11 And 2x 4y 24 And Hence Find The Value Of m
https://i.ytimg.com/vi/caKGprFnDYg/maxresdefault.jpg
When there is limited oxygen present during a combustion reaction carbon monoxide will be produced not carbon dioxide What is the formula for carbon monoxide Correct answer CO One way to separate carbon and hydrogen in methane is through a process called steam methane reforming This process involves reacting methane with steam at high
Incomplete oxidation of methane can lead to the formation of carbon monoxide and other carbon containing products This reaction typically occurs under conditions where there s limited Carbon turns to carbon monoxide This happens with any hydrocarbon We shall take methane as an example During incomplete combustion methane gas burns with a yellow flame unlike the
More picture related to 2x 5 12 8
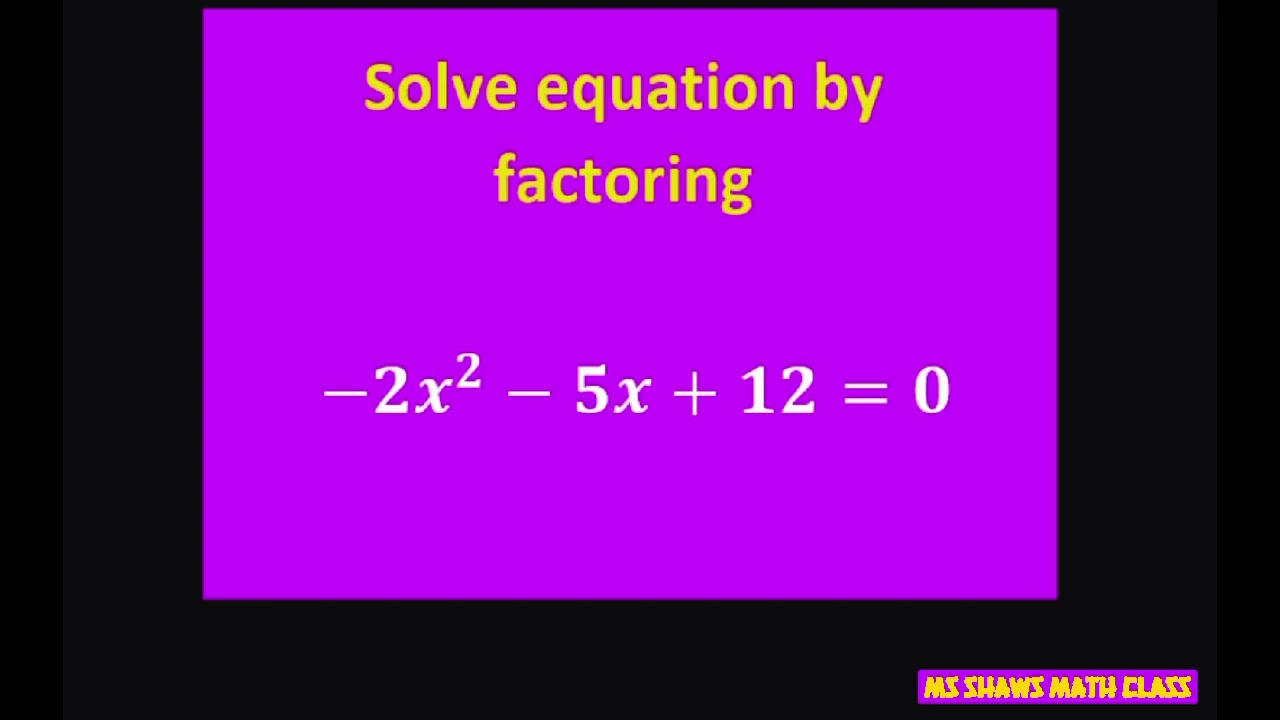
Solve Equation By Factoring 2x 2 5x 12 0 YouTube
https://i.ytimg.com/vi/VY2Y8PRYMTA/maxresdefault.jpg

Como Resolver Esta Ecuaci n 2 2x 5 5x 2 3x Y 3x 2x 5 5x 3x Y
https://es-static.z-dn.net/files/da7/2b7a7475f071873b1f9e98bf356c227e.jpg

Ayuda Es Para Ma ana c Brainly lat
https://es-static.z-dn.net/files/d03/05108a39ad21d34df7201d96ca8e5947.jpeg
Carbon monoxide causes drowsiness and affected people may fall unconscious or even die Learn about and revise fuels with this BBC Bitesize GCSE Chemistry Edexcel study guide When methane is burnt in air it reacts with oxygen to produce carbon dioxide and water vapor releasing heat energy in the process The chemical equation for the combustion
[desc-10] [desc-11]
Find The Match
https://wordwallscreens.azureedge.net/800/5b8be78b378342cb8ad75c3a1d87c6b9_0

Pakiii Ans Po Pls Thxxx Brainly ph
https://ph-static.z-dn.net/files/df4/880fb620ebc204ddb96a9fa4940f5fae.jpg

https://www.gauthmath.com › solution › d-Explain-why-it-is-not-safe-to...
Methane CH burns in the presence of oxygen O to produce carbon dioxide CO and water H O according to the following balanced chemical equation CH 2O CO 2H O

https://brainly.in › question
Answer When methane CH4 burns it reacts with oxygen gas to produce carbon dioxide and water The unbalanced equation for this reaction is CH4 g O2 g

MSI GeForce RTX 4060 Ti VENTUS 2X BLACK 8G
Find The Match

A Complete The Table Of Values For Y 2x 3 2 1 0 1 2 3 10 1 7

Graphing Linear Functions Examples Practice Expii

MSI GeForce RTX 4070 VENTUS 2X 12G OC Graphics Card MSI Global

3x 4 0 Estudiar

3x 4 0 Estudiar

Example 8 Multiply i x 4 And 2x 3 Algebra Class 8

Question 1 Find The Solution Of 2x 3 7 Chapter 2 Class 8

Example 17 Solve 5x 2 2x 7 2 3x 1 7 2 Chapter 2
2x 5 12 8 - One way to separate carbon and hydrogen in methane is through a process called steam methane reforming This process involves reacting methane with steam at high