Classification Of Medical Devices In Clinical Trials Important Information Canada has aligned the Workplace Hazardous Materials Information System WHMIS with the Globally Harmonized System of Classification and
What is a classification Classification is defined in Part 1 of the TDG Regulations as classification means for dangerous goods as applicable the shipping name the primary What is the fire tetrahedron To understand how to prevent fires it is important to know how a fire can occur
Classification Of Medical Devices In Clinical Trials

Classification Of Medical Devices In Clinical Trials
https://i.ytimg.com/vi/oOCzO-tLs2E/maxresdefault.jpg

Classification Of Medical Devices EU 2017 745 YouTube
https://i.ytimg.com/vi/oXgKchEMhJc/maxresdefault.jpg

Clinical Trials For Active Medical Devices YouTube
https://i.ytimg.com/vi/UxrkmaGRG0k/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AHUBoAC4AOKAgwIABABGCcgVChyMA8=&rs=AOn4CLBFBeP-ECzYKx7c_PC-CVq9Y9MzEg
The tertiary level of classification is species which is the most specific level and represents a group of organisms that can interbreed and produce fertile offspring MASS PACS
Asset classification is crucial for effective financial management Learn how to categorize assets into current non current tangible and intangible types Understand the Important Information Canada has aligned the Workplace Hazardous Materials Information System WHMIS with the Globally Harmonized System of Classification and
More picture related to Classification Of Medical Devices In Clinical Trials
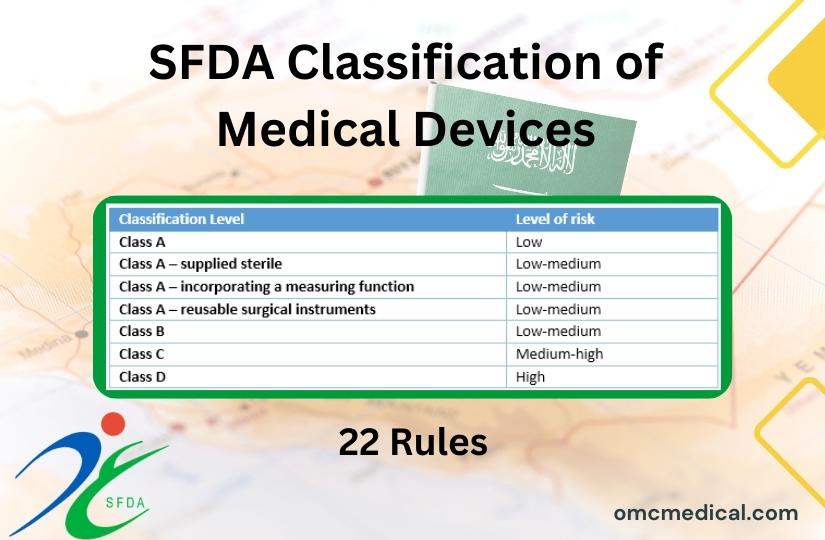
SFDA Classification Of Medical Devices 41 OFF
https://omcmedical.com/wp-content/uploads/2023/04/SFDA.jpg

The Importance Of The 4 Phases Of Clinical Trials Learn About The
https://i.pinimg.com/originals/f4/38/0d/f4380d90fc5e5022bed0e2d131c497bc.jpg
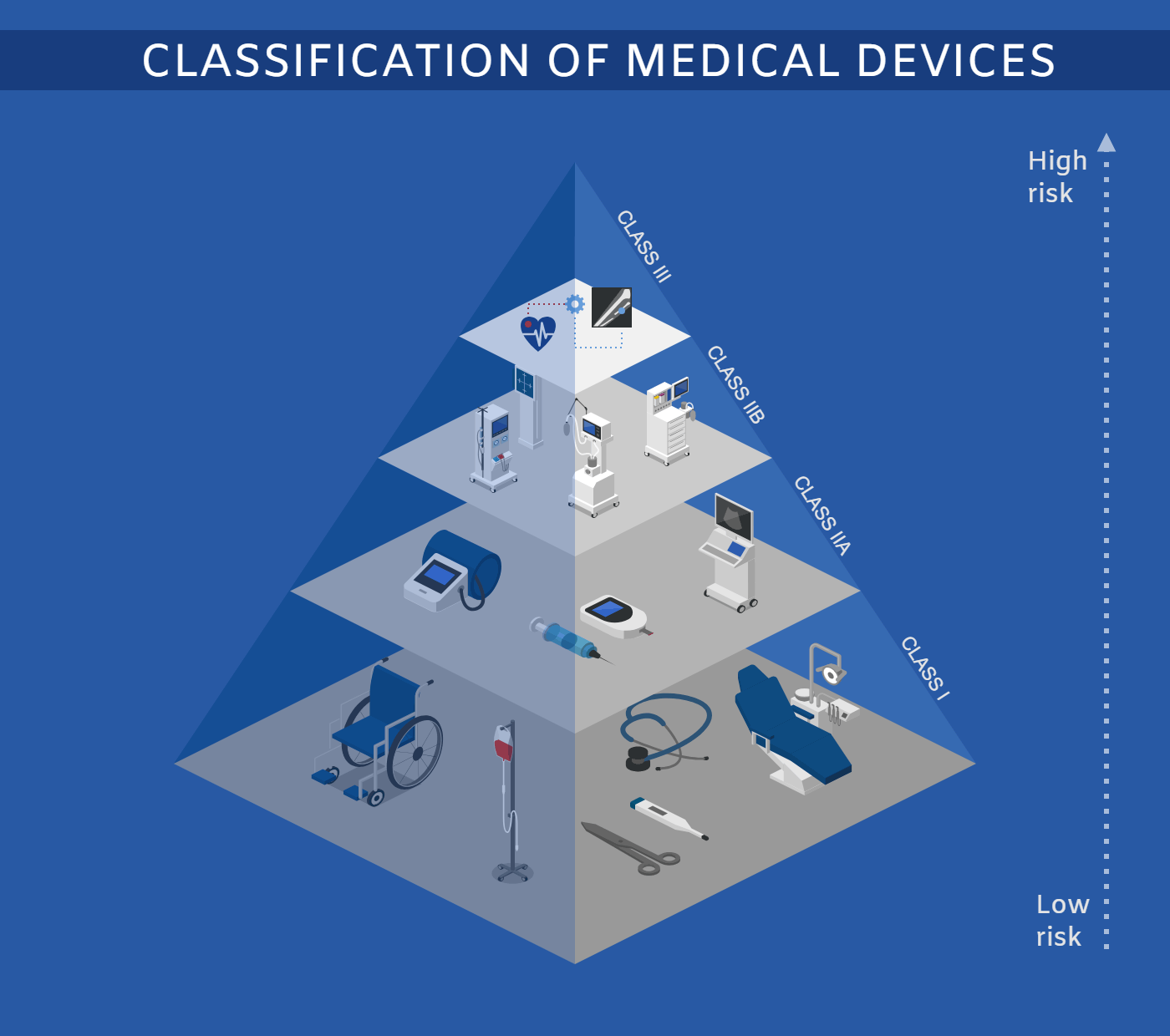
Icograms Templates Create Beautiful Isometric Diagrams Infographics
https://storage.icograms.com/templates/preview/classification-medical-devices.png
What are WHMIS classes or classifications WHMIS Workplace Hazardous Materials Information System uses classifications to group chemicals with similar properties What information is needed for classification Based on the definition for classification a competent person must determine the following before a classification can be assigned to a
[desc-10] [desc-11]
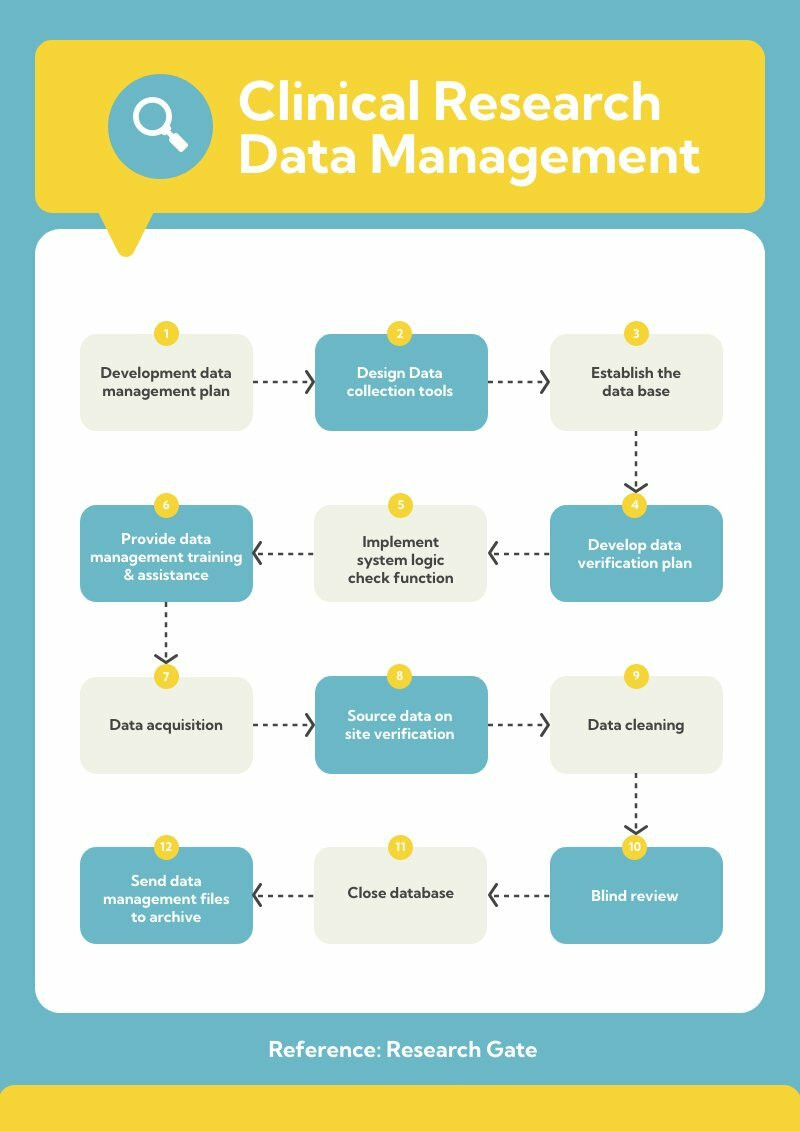
Turquoise Piktochart
https://c0.piktochart.com/v2/themes/2721-clinical-research-flowchart/snapshot/large.jpg
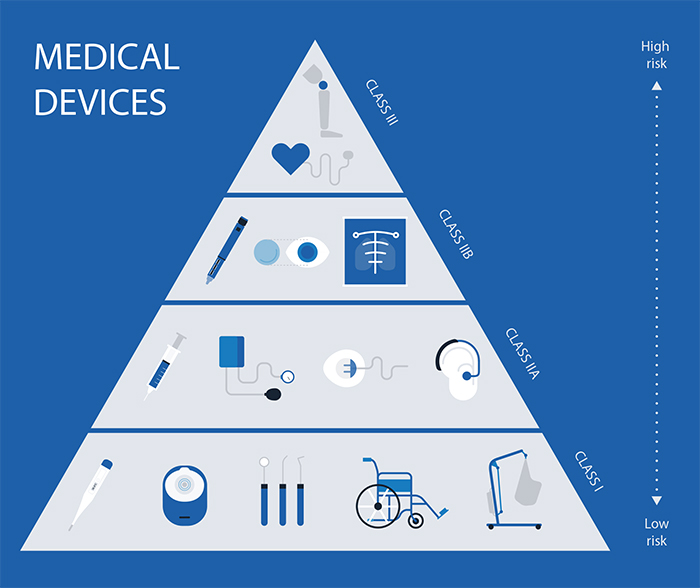
Overview Of Ireland s Registration Process RegDesk
https://www.regdesk.co/wp-content/uploads/2019/05/C5B3325D57D14B33B5CA0FA395952588.ashx_.jpeg

https://www.ccohs.ca › oshanswers › chemicals › whmis_ghs › hazard_c…
Important Information Canada has aligned the Workplace Hazardous Materials Information System WHMIS with the Globally Harmonized System of Classification and

https://www.ccohs.ca › oshanswers › legisl › tdg › tdg_classification.html
What is a classification Classification is defined in Part 1 of the TDG Regulations as classification means for dangerous goods as applicable the shipping name the primary
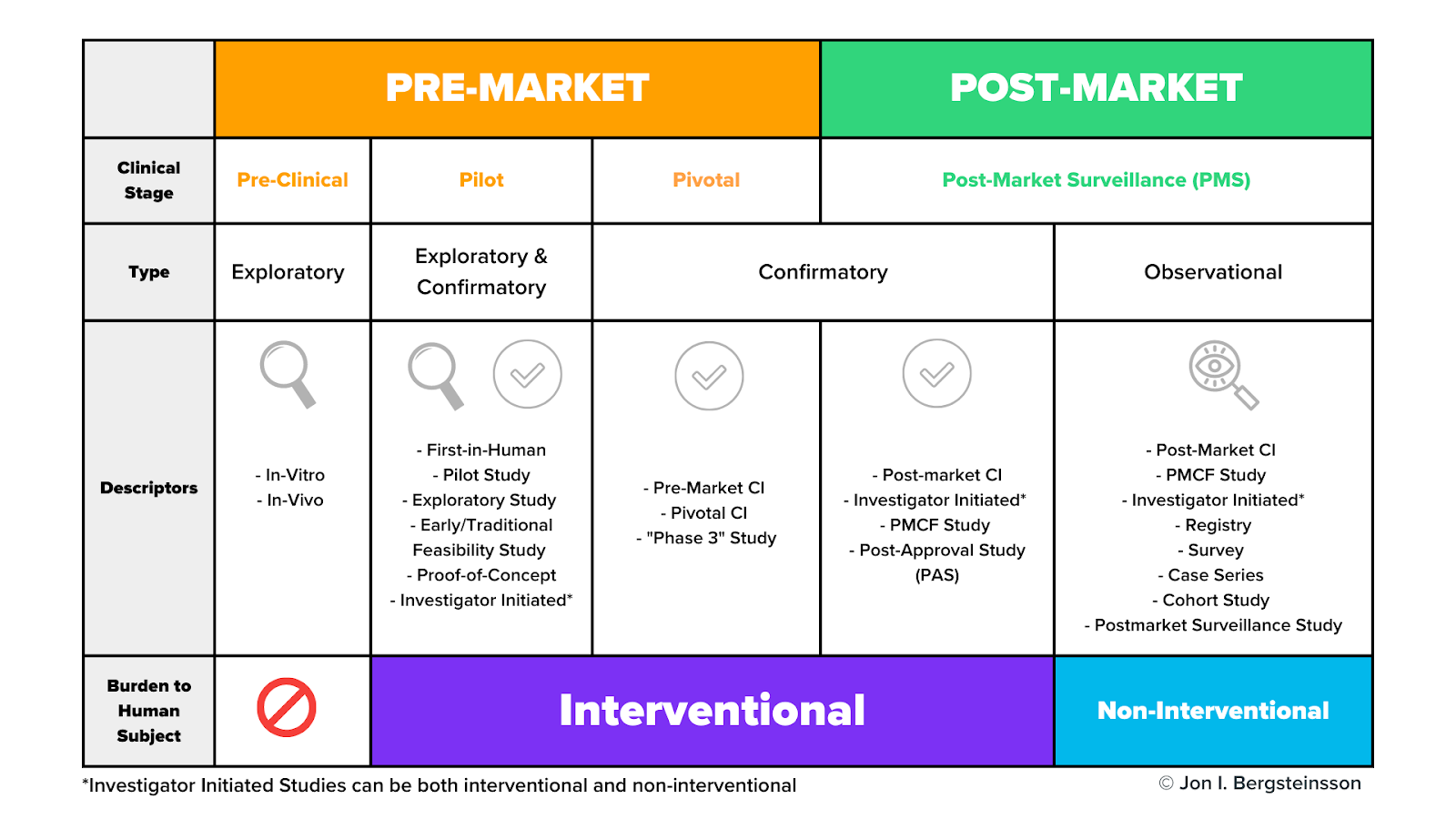
Study Design Penn Medicine Clinical Research Perelman School Of

Turquoise Piktochart

FDA Embraces Decentralized Clinical Trials DCT What You Need To Know
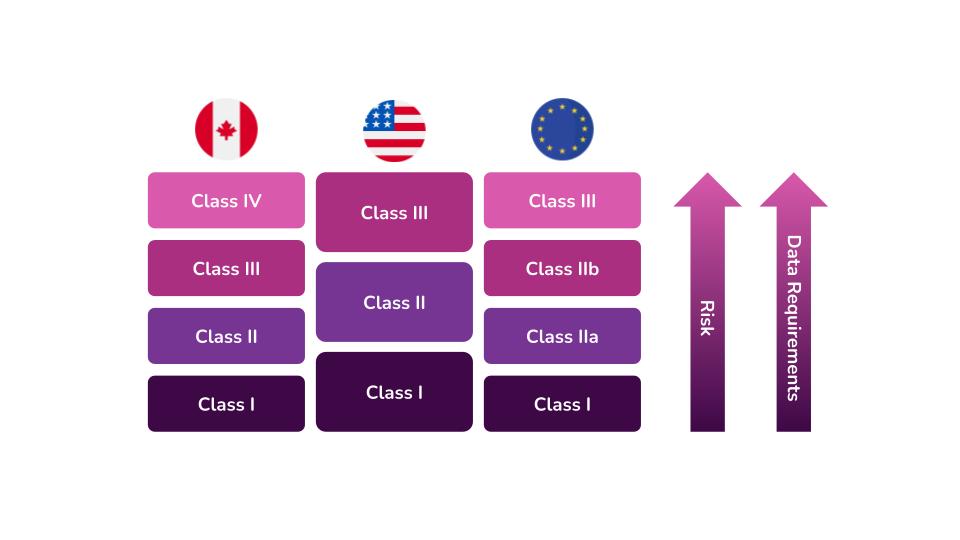
FDA Class II Medical Devices

Ca Biomedical Devices

Pharmacovigilance System Master File PVSMF Services Think i

Pharmacovigilance System Master File PVSMF Services Think i
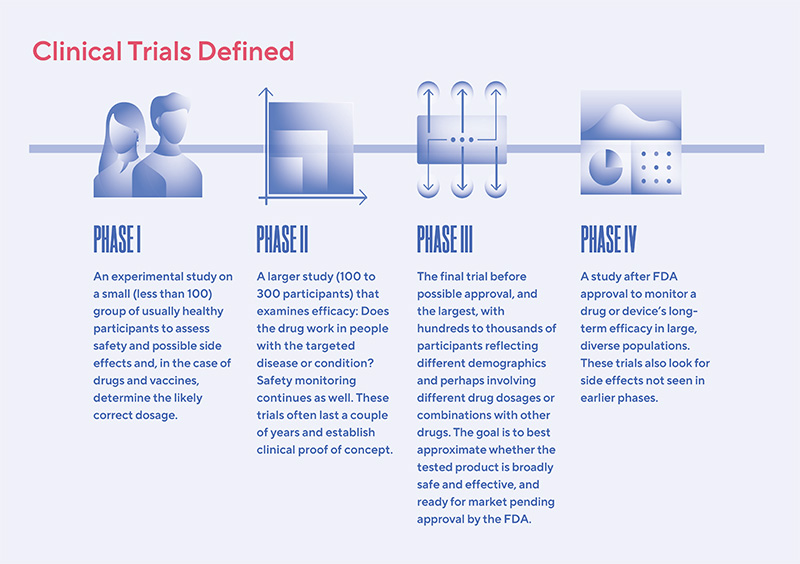
A New Phase

2024 Predictions For Clinical Trials Transforming Clinical Trials

19 17 Ppt Download
Classification Of Medical Devices In Clinical Trials - [desc-13]