Dilute Vs Concentrated Concentrated acid It is either pure acid or has a high concentration of acid Dilute acid A dilute acid is prepared by adding water to the concentrated acid and thus dilute acid has more water than the acid itself Thus a concentrated acid has more content of acid while dilute acid has a small amount of acid and more amount of water
Dilute HCl acid is stronger than highly concentrated acetic acid dilute HCl acid is stronger than The concept strong and weak acid is totally different from that of concentrated and dilute acid Whether an acid is strong or weak depends upon its Degree of Dissociation If degree of dissociation of an acid is more it is a strong acid eg HCl H2SO4 HNO3 If degree of dissociation is less then the acid is weak eg Carbonic acid
Dilute Vs Concentrated

Dilute Vs Concentrated
https://i.ytimg.com/vi/V6-mlh9xRjg/maxresdefault.jpg

Dilute And Concentrated Solution YouTube
https://i.ytimg.com/vi/VzhHK-0o3X8/maxresdefault.jpg

6 Dilute Vs Concentrated Urine YouTube
https://i.ytimg.com/vi/gyCTygweAHk/maxresdefault.jpg
A dilute acid is not a very concentrated one When adding water you can dilute an acid solution even further Strong and weak characterize the tendency of the acid to dissociate regardless of concentration into an aqueous solution Concentrated acid is a solution with a high concentration of hydrogen ions in molars ii Dilute HCl cannot be concentrated beyond 22 2 by distillation iii Dilute acids are stronger electrolytes as compared to concentrated acid iv Liquid ammonia has no action on litmus while liquor ammonia has an effect v Ammonia is not collected over water vi Pure nitric acid does not dissolve gold or platinum but aqua regia
With barium chloride solution added to dilute sulphuric acid gives white precipitate of BaSO 4 insoluble in conc HCI On the other hand dil HCI does not give any precipitate BaCl 2 BaCl 2 aq H 2 SO 4 aq BaSO 4 s 2 HCI aq Barium chloride Sulphuric acid Barium sulphate Hydrochloric acid Q The molar conductivity of acetic acid solution at infinite dilution is 390 7 Calculate the moalr conductivity of 0 01M acetic acid solution given that the dissociation of acetic acid is
More picture related to Dilute Vs Concentrated

Solutions Concentrated Vs Dilute YouTube
https://i.ytimg.com/vi/GzERcob-DEw/maxresdefault.jpg
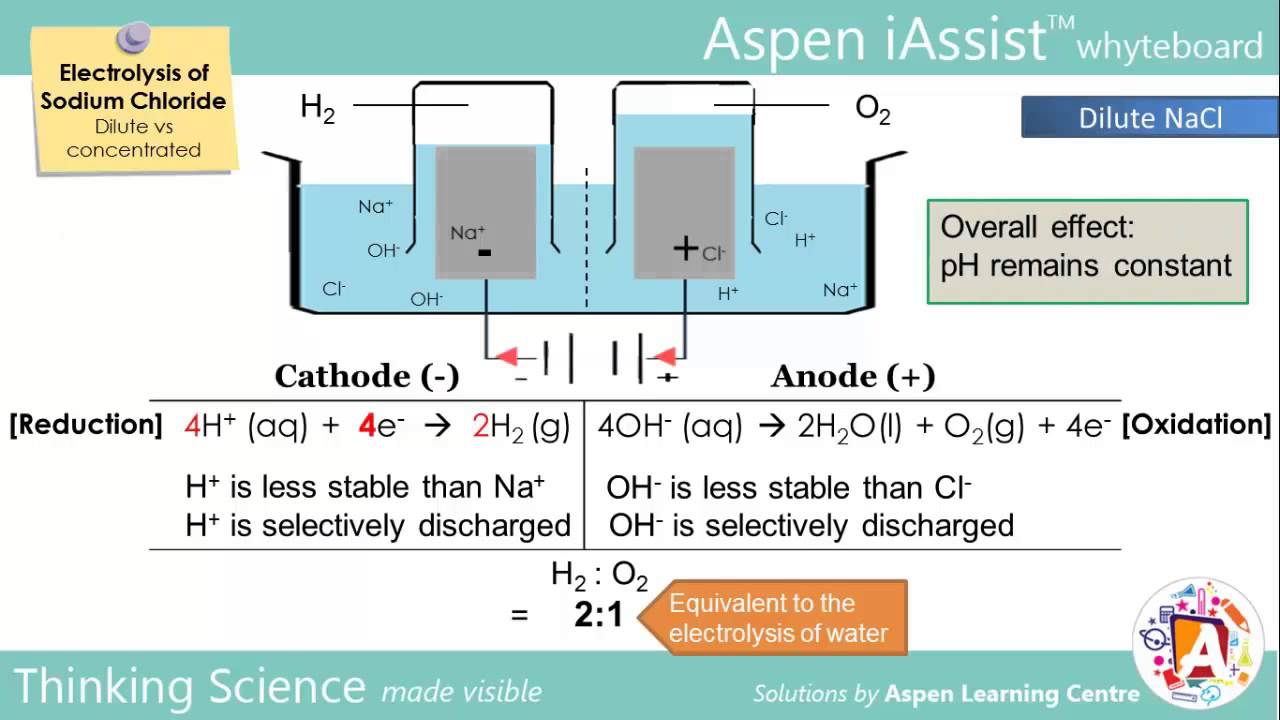
Electrolysis Of NaCl dilute Vs Concentrated YouTube
https://i.ytimg.com/vi/1FDzt5VP50c/maxresdefault.jpg

What Is Dilute Solution Chemistry YouTube
https://i.ytimg.com/vi/eVhJnFpRqG0/maxresdefault.jpg
Q Statement I Osmotic pressure of a dilute solution is inversely proportional to its concentration provided temperature remains constant Statement II Osmotic pressure is a colligative properly and depends upon the number of moles of solute dissolved in a definite volume of the solution iii Hydrochloric acid cannot be concentrated above 22 2 by boiling iv HCl gas does not conduct electricity but hydrochloric acid conducts electricity v Only a very dilute solution of hydrogen chloride in water can be concentrated by boiling the solution while a very concentrated solution would become less concentrated when boiled
[desc-10] [desc-11]

Dilute Concentrated And Saturated Solution YouTube
https://i.ytimg.com/vi/bu0TNjIcVAM/maxresdefault.jpg

What Is A Negative Dilute Drug Test Result YouTube
https://i.ytimg.com/vi/yTOGhiekn14/maxresdefault.jpg

https://byjus.com › question-answer › explain-why-does-dilute-sulphuric …
Concentrated acid It is either pure acid or has a high concentration of acid Dilute acid A dilute acid is prepared by adding water to the concentrated acid and thus dilute acid has more water than the acid itself Thus a concentrated acid has more content of acid while dilute acid has a small amount of acid and more amount of water

https://byjus.com › question-answer › dilute-hcl-acid-is-stronger-than-hig…
Dilute HCl acid is stronger than highly concentrated acetic acid dilute HCl acid is stronger than

Forming Urine Influencing Factors Forming Dilute Urine Forming

Dilute Concentrated And Saturated Solution YouTube

Aqueous Dilute And Concentrated Solution General Science Lecture
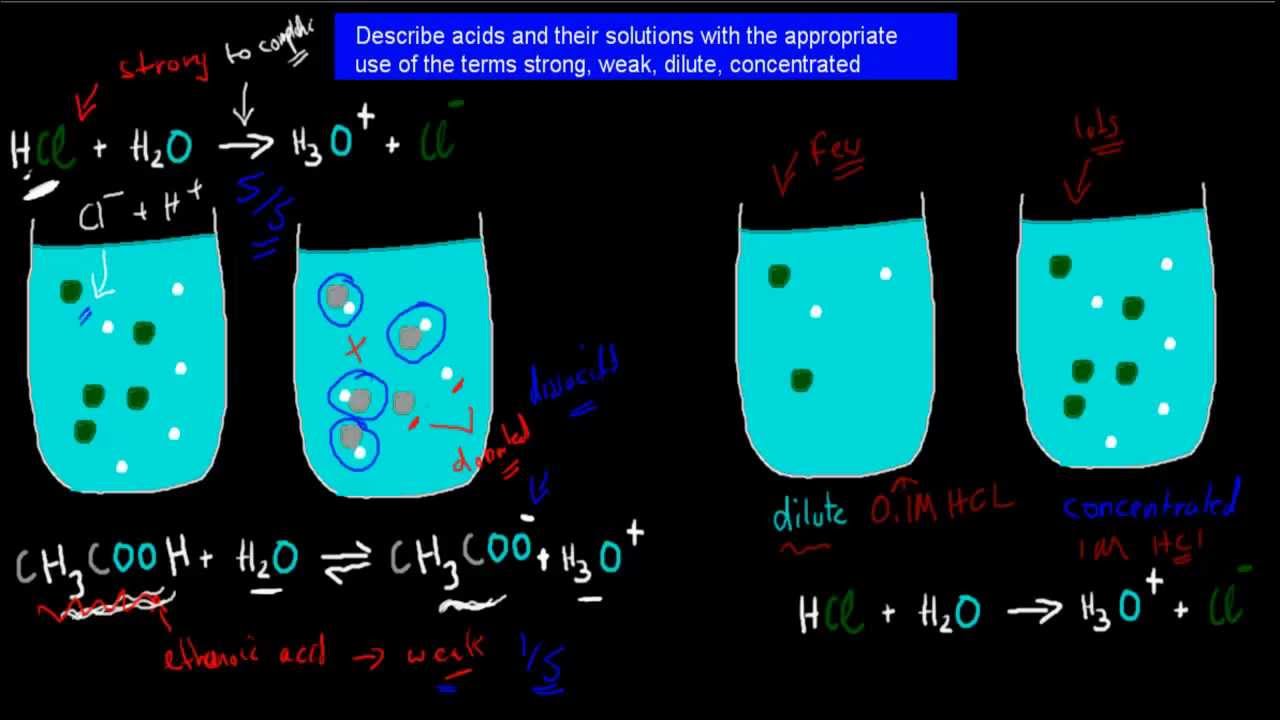
3 Strong Weak Dilute And Concentrated Acids HSC Chemistry YouTube
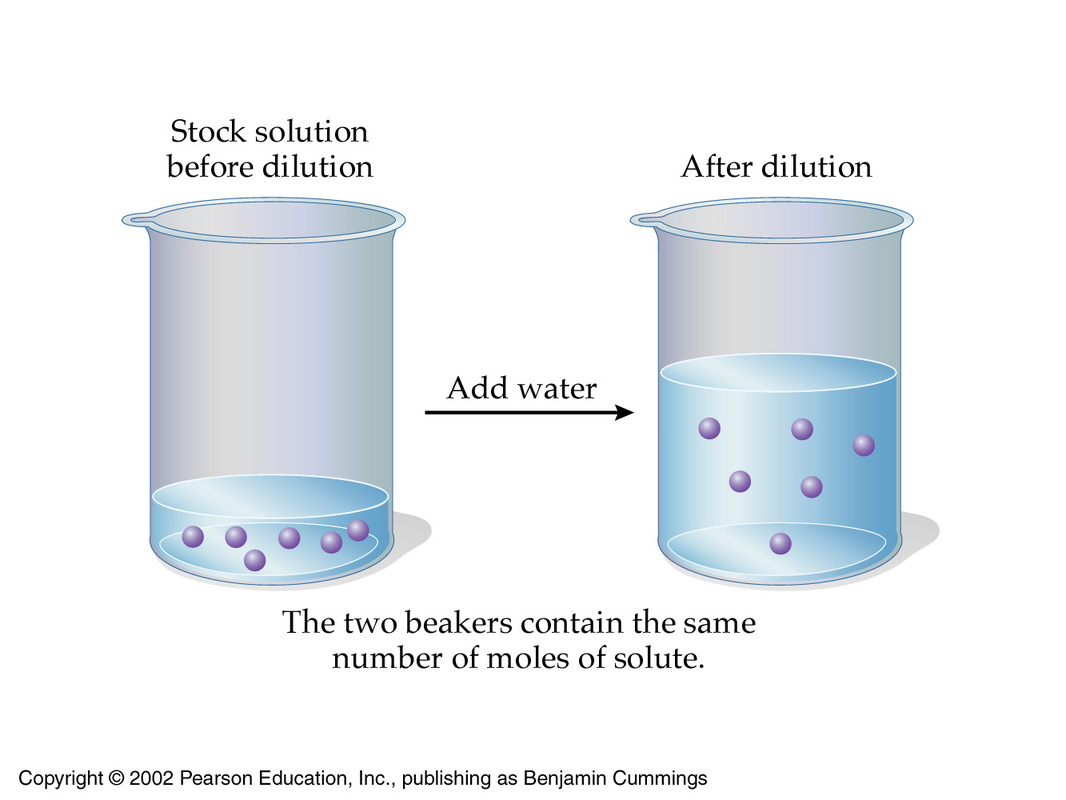
Molarity Acid And Bases For Dummies
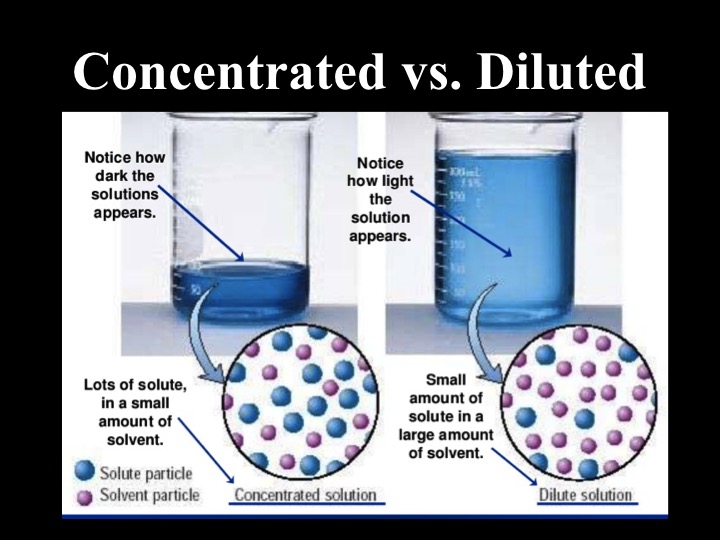
Concentrated Vs Diluted Y9 Science

Concentrated Vs Diluted Y9 Science

Concentrated Vs Diluted Y9 Science
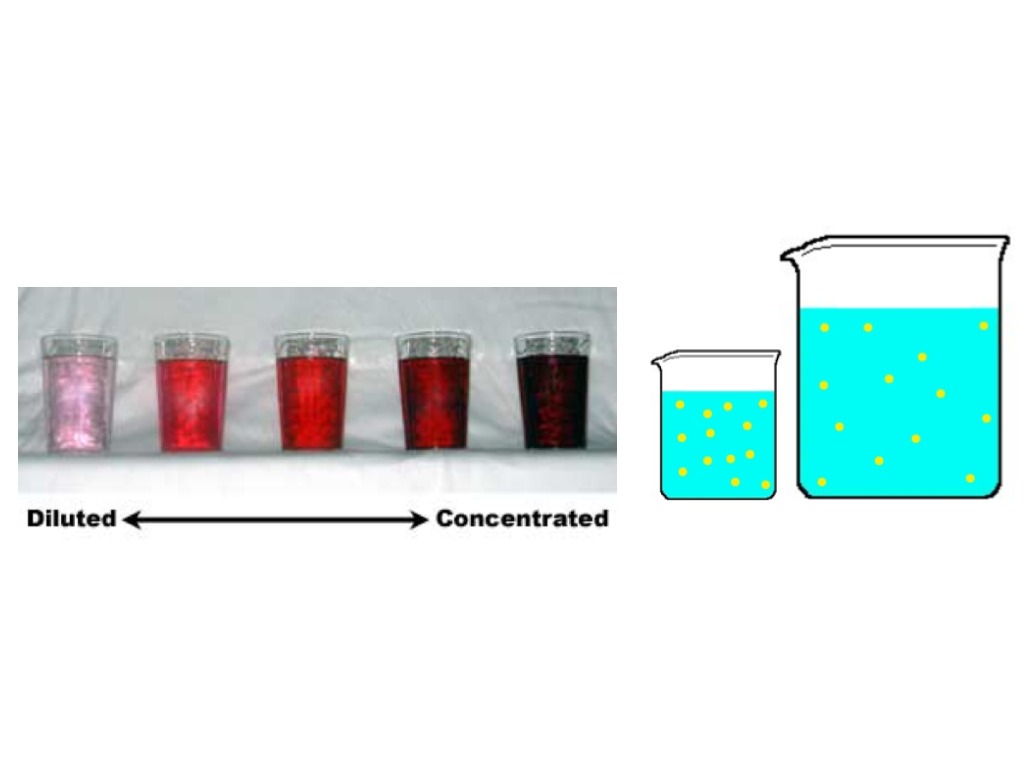
Concentration Solution Vs Diluted Solution Science ShowMe
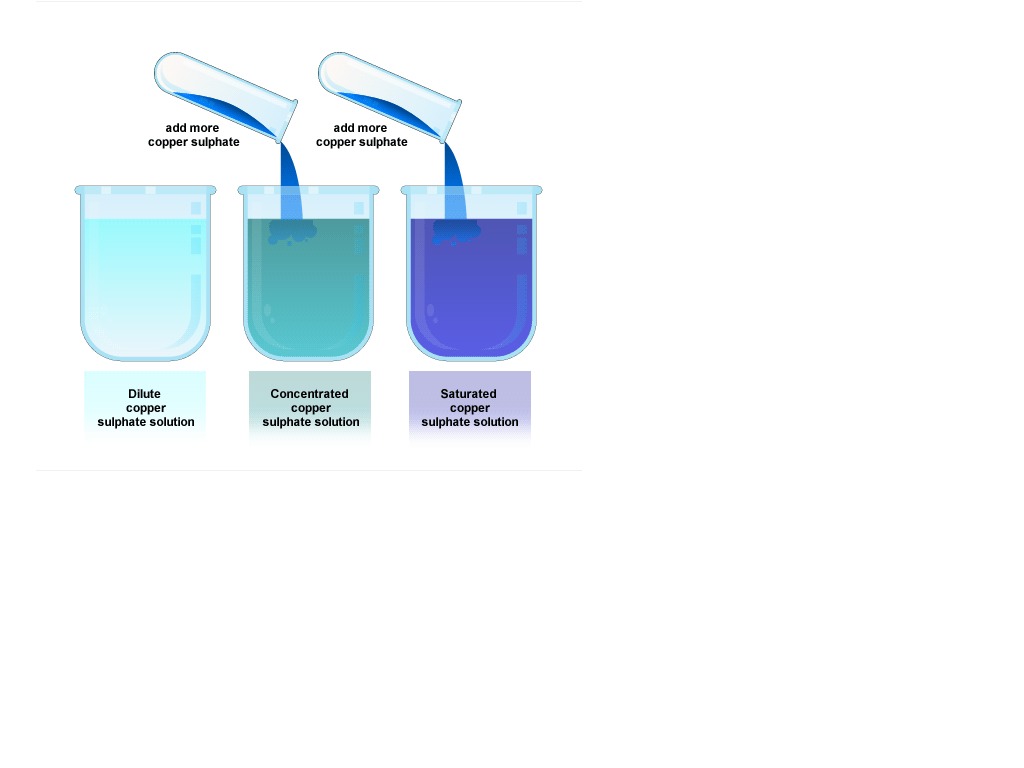
Concentrated Vs Diluted Solution Science ShowMe
Dilute Vs Concentrated - Q The molar conductivity of acetic acid solution at infinite dilution is 390 7 Calculate the moalr conductivity of 0 01M acetic acid solution given that the dissociation of acetic acid is