How Are Medical Devices Classified In The Eu The development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of WHO
This handbook the result of extensive international consultation and collaboration provides comprehensive guidance on safe efficient and environmentally sound methods for The data presented in the Global Health Workforce Statistics database are processed data extracts of the national reporting in the NHWA data platform Complementing the national
How Are Medical Devices Classified In The Eu

How Are Medical Devices Classified In The Eu
https://i.pinimg.com/originals/44/01/19/440119dd36aa51fc2dd473d08103b273.jpg

What Is The Difference Between Promotional And Classified 48 OFF
https://www.themediaant.com/blog/wp-content/uploads/2022/12/History-of-Advertisement-in-India-1-2.jpg

Medical Transparent PNG All
https://www.pngall.com/wp-content/uploads/15/Medical-Transparent.png
Substandard and falsified medical products Suicide prevention Socio political determinants Sodium reduction Soil transmitted helminth infections Spinal cord injury Sporotrichosis Substandard and falsified medical products Sugars and dental caries Suicide Syphilis T
Sexual health cannot be defined understood or made operational without a broad consideration of sexuality which underlies important behaviours and outcomes related to Coronavirus disease COVID 19 The virus can spread from an infected person s mouth or nose in small liquid particles when they cough sneeze speak sing or breathe
More picture related to How Are Medical Devices Classified In The Eu
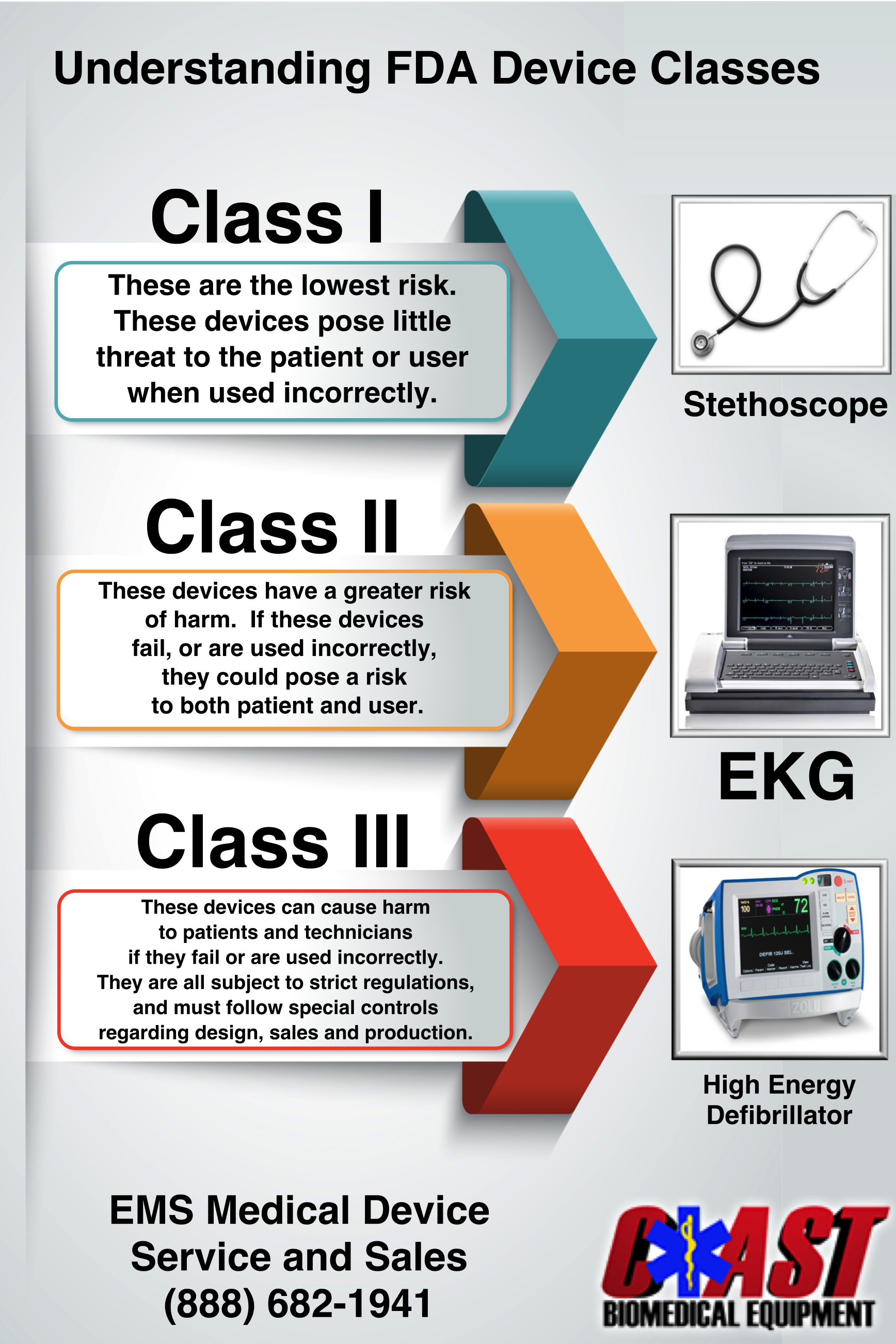
Blog Coast Biomedical Equipment
https://coastbiomed.com/wp-content/uploads/2016/01/CoastInfographic.png

Classaified Telegraph
https://c8.alamy.com/comp/MTB113/top-secret-document-declassified-confidential-information-secret-text-non-public-information-sheet-of-paper-with-classified-information-MTB113.jpg

Data And IT Resource Classification Standard Overview Information
https://security.berkeley.edu/sites/default/files/protectionlevelsummary_0.jpeg
This WHO questions and answers page looks at how health related disinformation has emerged as a threat to public health and safety It explains in a general way how There are few truer snapshots of a country s wellbeing than its health statistics While broad economic indicators such as Gross Domestic Product may skew impressions of individual
[desc-10] [desc-11]
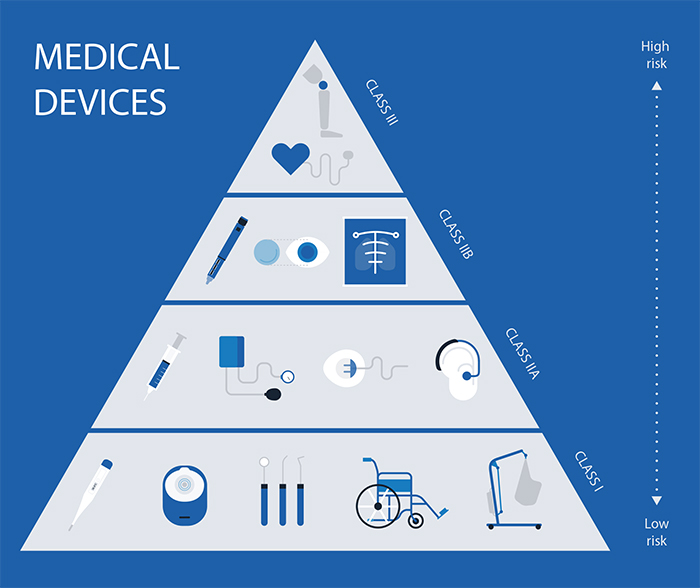
Overview Of Ireland s Registration Process RegDesk
https://www.regdesk.co/wp-content/uploads/2019/05/C5B3325D57D14B33B5CA0FA395952588.ashx_.jpeg

Ce Symbol
https://i.ytimg.com/vi/IFXL_Bup-90/maxresdefault.jpg

https://www.who.int › publications › who-guidelines
The development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of WHO

https://www.who.int › publications › item
This handbook the result of extensive international consultation and collaboration provides comprehensive guidance on safe efficient and environmentally sound methods for

Trump Gif 2024 Trump Jr Flannel

Overview Of Ireland s Registration Process RegDesk

EU Medical Device Regulations APCER Life Sciences
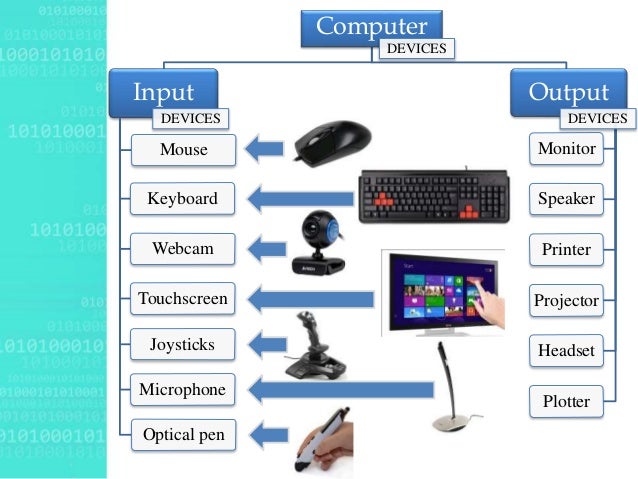
Output Device Computer Projects Input Devices
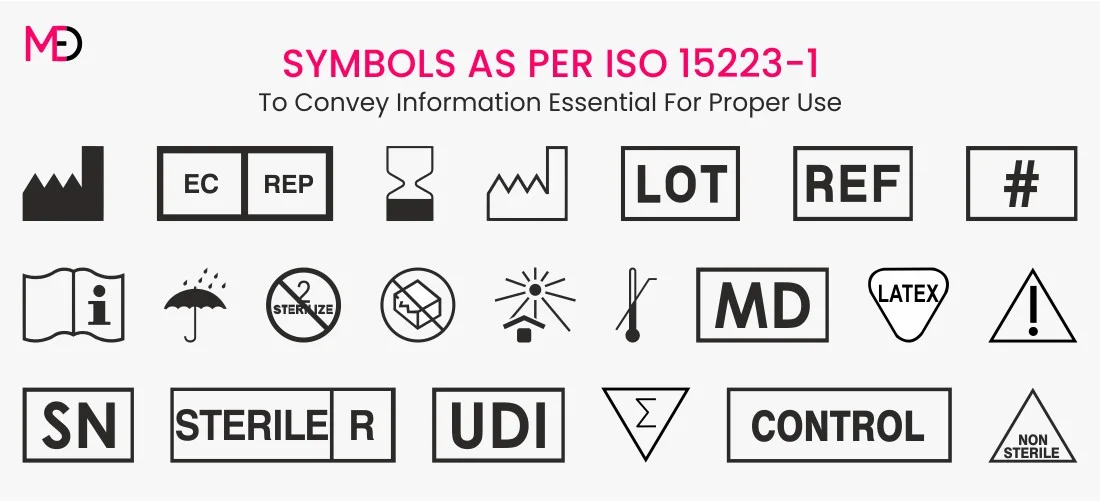
EU MDR Medical Device Labeling Requirements A Complete Guide

Electronic Items List

Electronic Items List
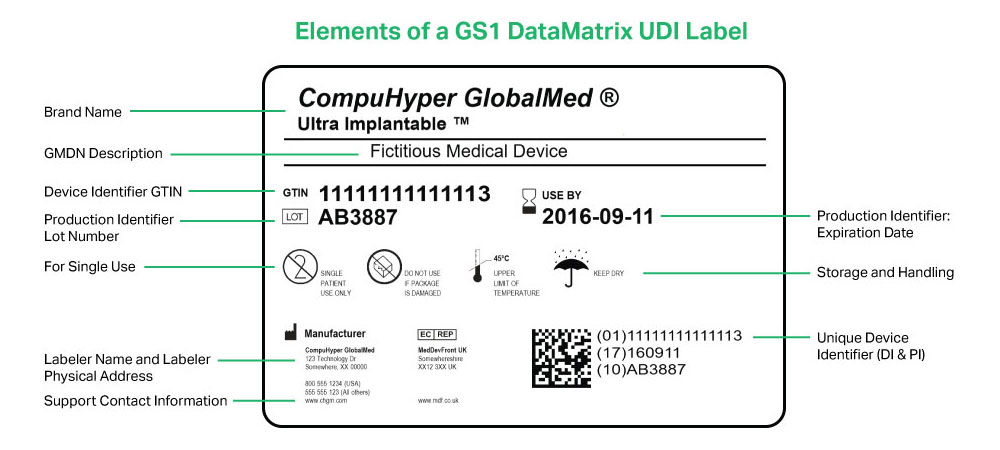
FDA UDI NiceLabel
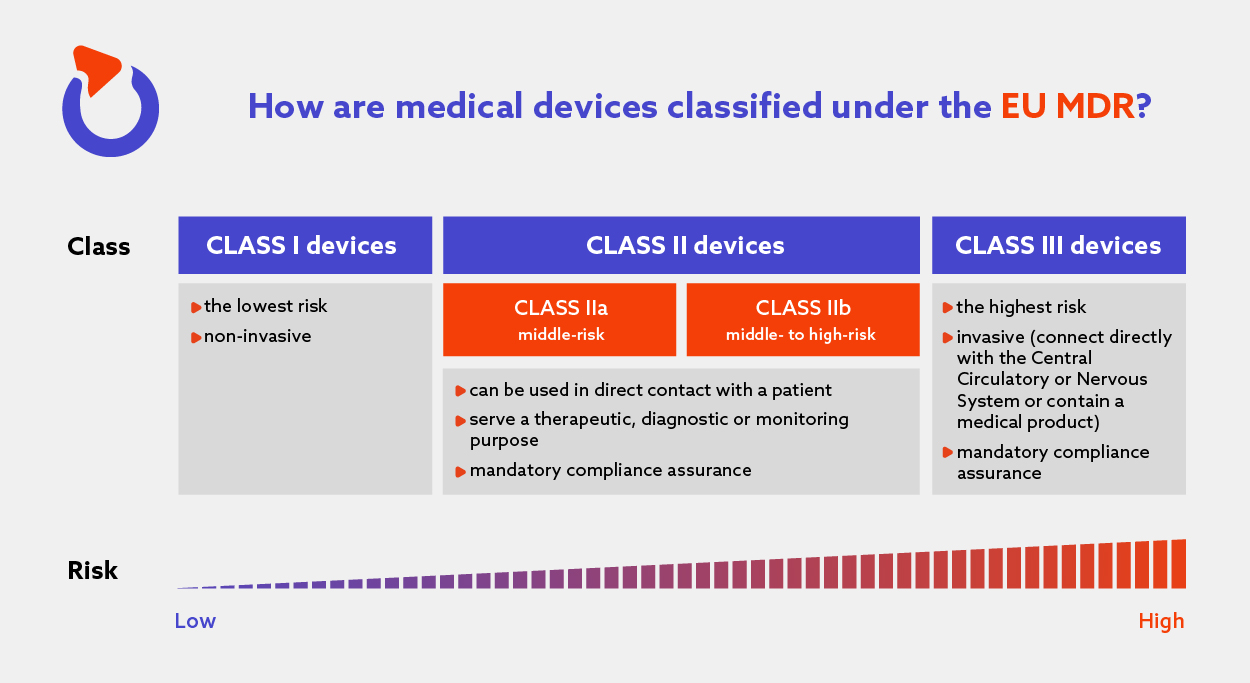
The Complete Guide To EU Medical Device Regulation Spyrosoft

11 Most Innovative Medical Devices Of 2017 Legacy MedSearch Medical
How Are Medical Devices Classified In The Eu - Substandard and falsified medical products Suicide prevention Socio political determinants