How Are Medical Devices Classified This handbook the result of extensive international consultation and collaboration provides comprehensive guidance on safe efficient and environmentally sound methods for
Sexual health cannot be defined understood or made operational without a broad consideration of sexuality which underlies important behaviours and outcomes related to The United Nations agency working to promote health keep the world safe and serve the vulnerable
How Are Medical Devices Classified

How Are Medical Devices Classified
https://remmed.com/wp-content/uploads/2023/05/Remington-Logo-R-Dark.png
Adhithiyan LK On LinkedIn How Are Medical Devices Classified Welcome
https://media.licdn.com/dms/image/v2/D5605AQFUTGKPD3Wy9Q/videocover-high/videocover-high/0/1727763153321?e=2147483647&v=beta&t=B2hQ8EnpH8ip2WYOjKLB1AXQvMzEWrCD2UNfHwX0wIo
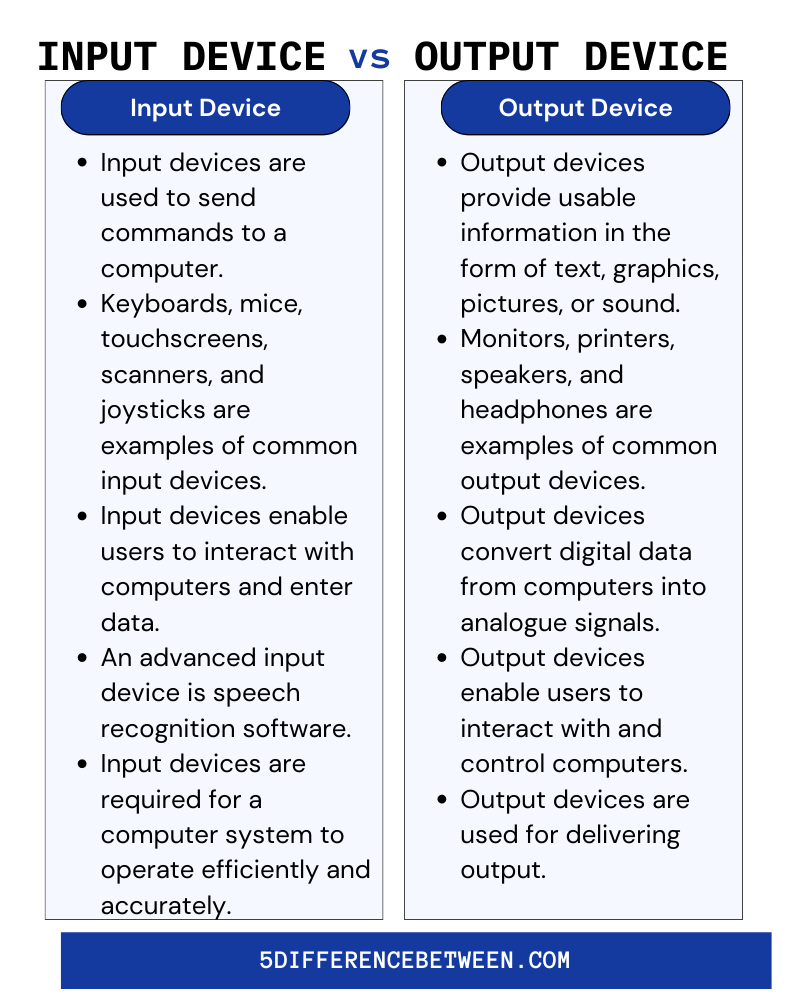
Difference Between Input And Output Devices TutorialsMate 55 OFF
https://5differencebetween.com/wp-content/uploads/2023/02/Input-Vs-Output-Device.png
The data presented in the Global Health Workforce Statistics database are processed data extracts of the national reporting in the NHWA data platform Complementing the national There are few truer snapshots of a country s wellbeing than its health statistics While broad economic indicators such as Gross Domestic Product may skew impressions of individual
This WHO questions and answers page looks at how health related disinformation has emerged as a threat to public health and safety It explains in a general way how Substandard and falsified medical products Suicide prevention Socio political determinants
More picture related to How Are Medical Devices Classified

Medical Transparent PNG All
https://www.pngall.com/wp-content/uploads/15/Medical-Transparent.png
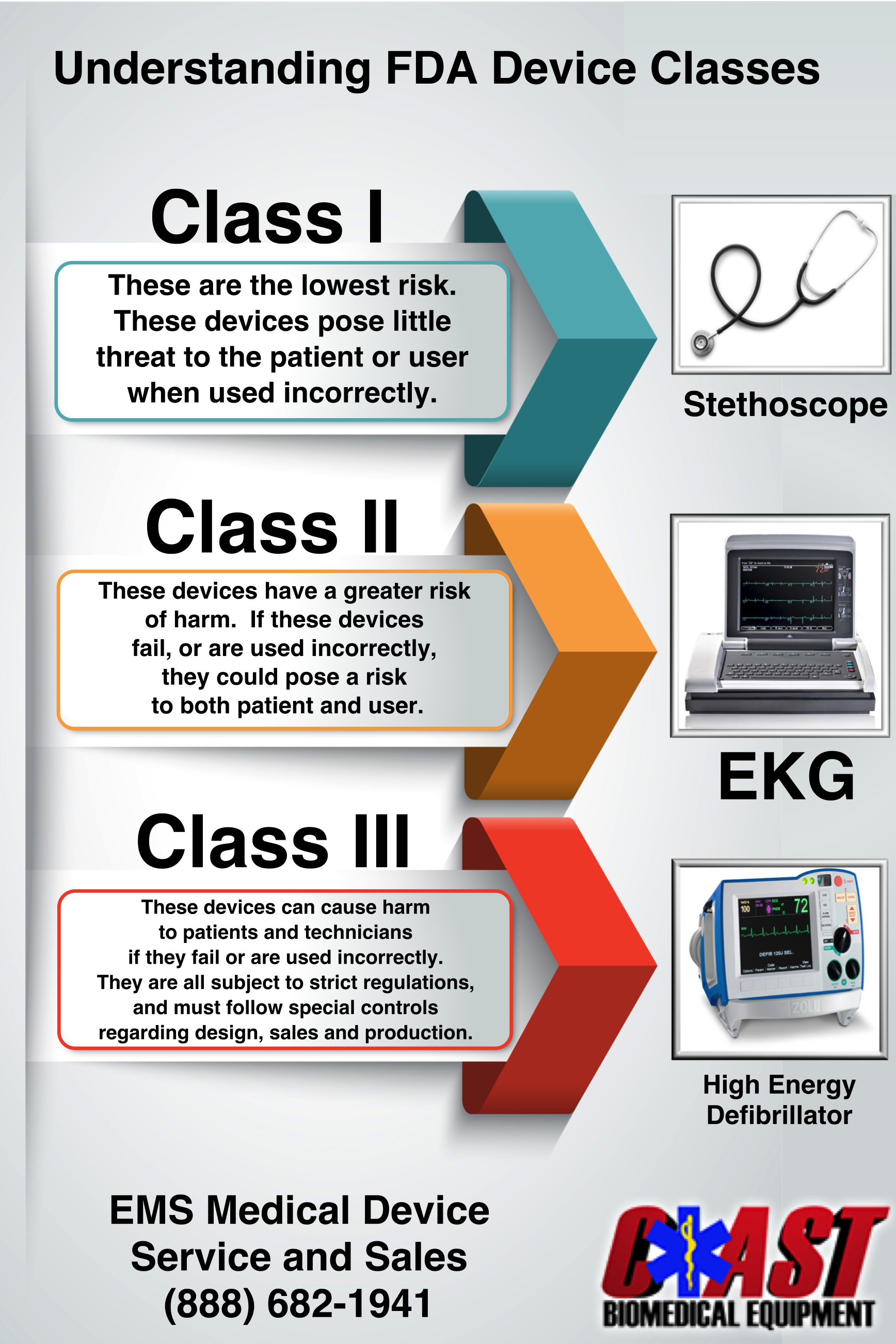
Blog Coast Biomedical Equipment
http://coastbiomed.com/wp-content/uploads/2016/01/CoastInfographic.png

Cervical Collar Hard Glorified Ortho
https://glorifiedortho.com/wp-content/uploads/2023/10/cervical-collar-hard3-scaled.jpg
Together for a healthier world WHO offer professionals a unique opportunity to contribute to saving lives and support people s health worldwide Good Manufacturing Practices GMP also referred to as cGMP or current Good Manufacturing Practice is the aspect of quality assurance that ensures that medicinal products are
[desc-10] [desc-11]
Medical Devices
https://laegemiddelstyrelsen.dk/en/~/media/C5B3325D57D14B33B5CA0FA395952588.ashx

Medical Devices
https://www.who.int/images/default-source/access-to-medicines/who_052221.tmb-1024v.jpg?sfvrsn=c2f256ce_2

https://www.who.int › publications › item
This handbook the result of extensive international consultation and collaboration provides comprehensive guidance on safe efficient and environmentally sound methods for

https://www.who.int › health-topics › sexual-health
Sexual health cannot be defined understood or made operational without a broad consideration of sexuality which underlies important behaviours and outcomes related to
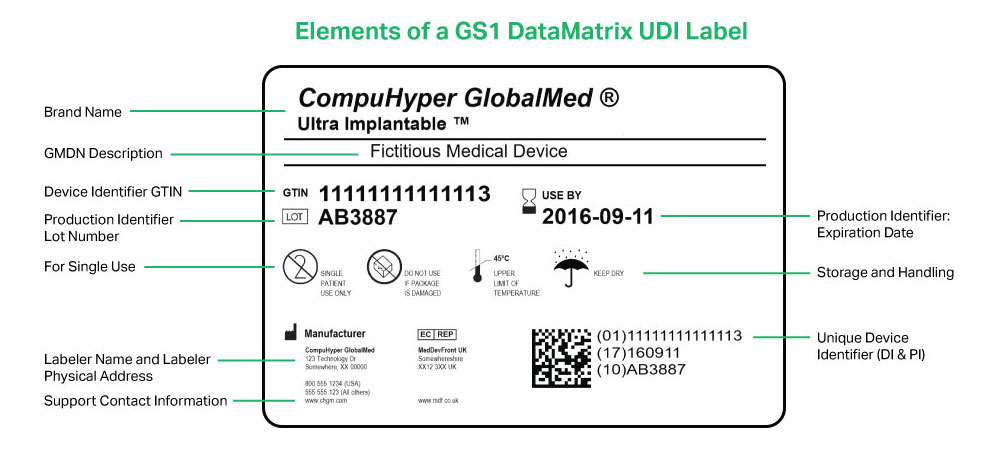
FDA UDI Compliance NiceLabel
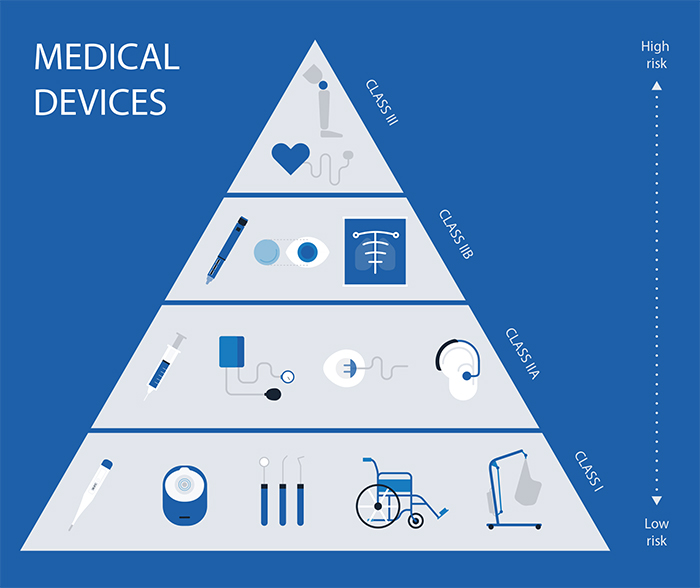
Medical Devices

Selected Medical Devices And Their Australian Classification Download
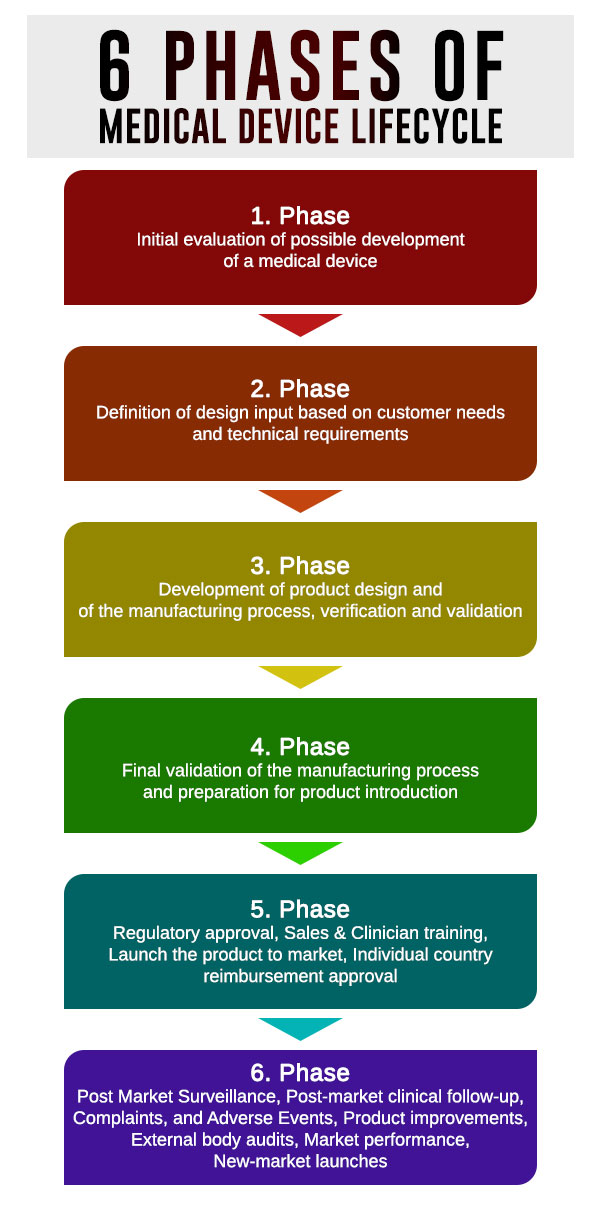
What Is Medical Devices Lifecycle QmsWrapper
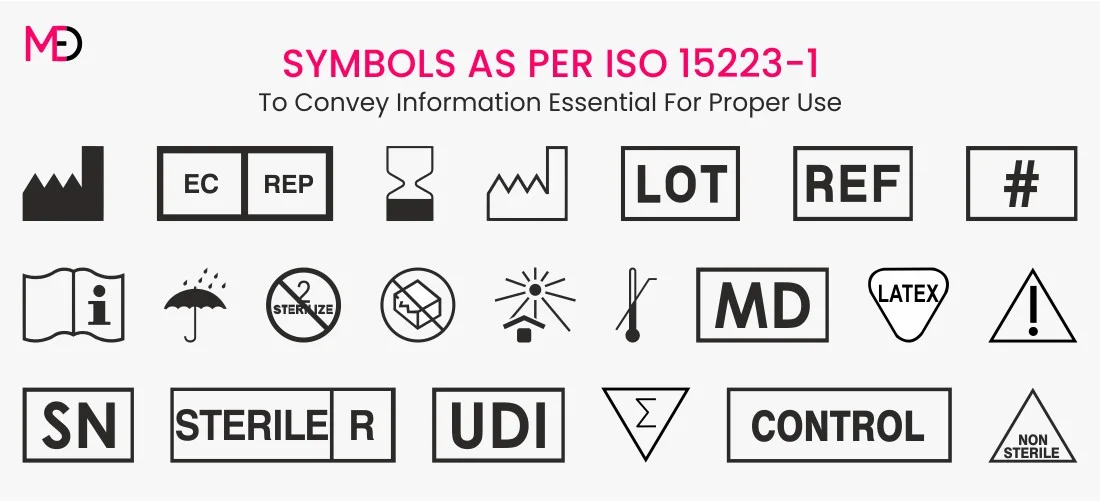
EU MDR Medical Device Labeling Requirements A Complete Guide

Electronic Items List

Electronic Items List

11 Most Innovative Medical Devices Of 2017 Legacy MedSearch Medical

Legacy Devices In The MDR Tracekey Solutions GmbH

PDF Exemple D une le L le De La R union PDF T l charger Download
How Are Medical Devices Classified - [desc-13]
