What Is The Meaning Of Average Kinetic Energy Kinetic energy form of energy that an object or a particle has by reason of its motion Kinetic energy is a property of a moving object or particle
Average kinetic energy of a molecule An important property of molecules in a gas is their average translational kinetic energy For a gas translational kinetic energy is defined What is average kinetic energy According to kinetic molecular theory the average kinetic energy of gas particles is dependent upon the temperature of the gas The average kinetic energy of a
What Is The Meaning Of Average Kinetic Energy

What Is The Meaning Of Average Kinetic Energy
https://i.ytimg.com/vi/Lc5TgTdNfzM/maxresdefault.jpg

How To Calculate Kinetic Energy Simple Formula Easy Examples
https://i.ytimg.com/vi/aFZPWL8RqYw/maxresdefault.jpg

Calculate Average Kinetic Energy Total Transitional Kinetic Energy
https://i.ytimg.com/vi/P01v_ai8b_k/maxresdefault.jpg
The average kinetic energy formula calculates the average amount of motion that a set of particles possesses It is closely related to the concepts of temperature mass and The formula for average kinetic energy is 1 2 m v 2 where m is the mass of the particle v is its velocity and 1 2 is a constant The units of average kinetic energy are joules
The measure of average kinetic energy is the temperature which represents the mean kinetic energy of particles in a system It is a statistical measure that quantifies the Learn about kinetic energy and its types including translational rotational vibrational thermal and electrical Discover examples formulas and more in this comprehensive article on kinetic energy
More picture related to What Is The Meaning Of Average Kinetic Energy

Average Kinetic Energy Vs Molecular Velocity Speed Definition
https://i.ytimg.com/vi/Vt6NzUaCT4o/maxresdefault.jpg

How To Calculate The Average Translational Kinetic Energy Of Molecules
https://i.ytimg.com/vi/bg5d5BFANVU/maxresdefault.jpg

10 3a Understanding How Average Molecular Kinetic Energy Scales With
https://i.ytimg.com/vi/aC3TMqMU3Vk/maxresdefault.jpg
Mean kinetic energy measures the average energy of molecules in motion reflecting their temperature It relates to root mean square velocity which considers the Average kinetic energy refers to the average energy possessed by the particles of a substance due to their motion It is directly related to the temperature of the substance
Average Kinetic Energy At any given temperature not all of the particles of a sample of matter have the same kinetic energy Instead the particles display a wide range of kinetic energies Most of the particles have a kinetic energy Average Kinetic Energy At any given temperature not all of the particles of a sample of matter have the same kinetic energy Instead the particles display a wide range of

Aleks Understanding How Average Molecular Kinetic Energy Scales With
https://i.ytimg.com/vi/QyDj10BB96g/maxresdefault.jpg
Potential And Kinetic Energy By Science
https://lookaside.fbsbx.com/lookaside/crawler/media/?media_id=493824054359482&get_thumbnail=1

https://www.britannica.com › science › kin…
Kinetic energy form of energy that an object or a particle has by reason of its motion Kinetic energy is a property of a moving object or particle

https://www.savemyexams.com › a-level › physics › cie › ...
Average kinetic energy of a molecule An important property of molecules in a gas is their average translational kinetic energy For a gas translational kinetic energy is defined

Rumus Energi Kinetik Rumuspedia

Aleks Understanding How Average Molecular Kinetic Energy Scales With

This Image Shows A Number Of Different Examples Where Kinetic Energy Is
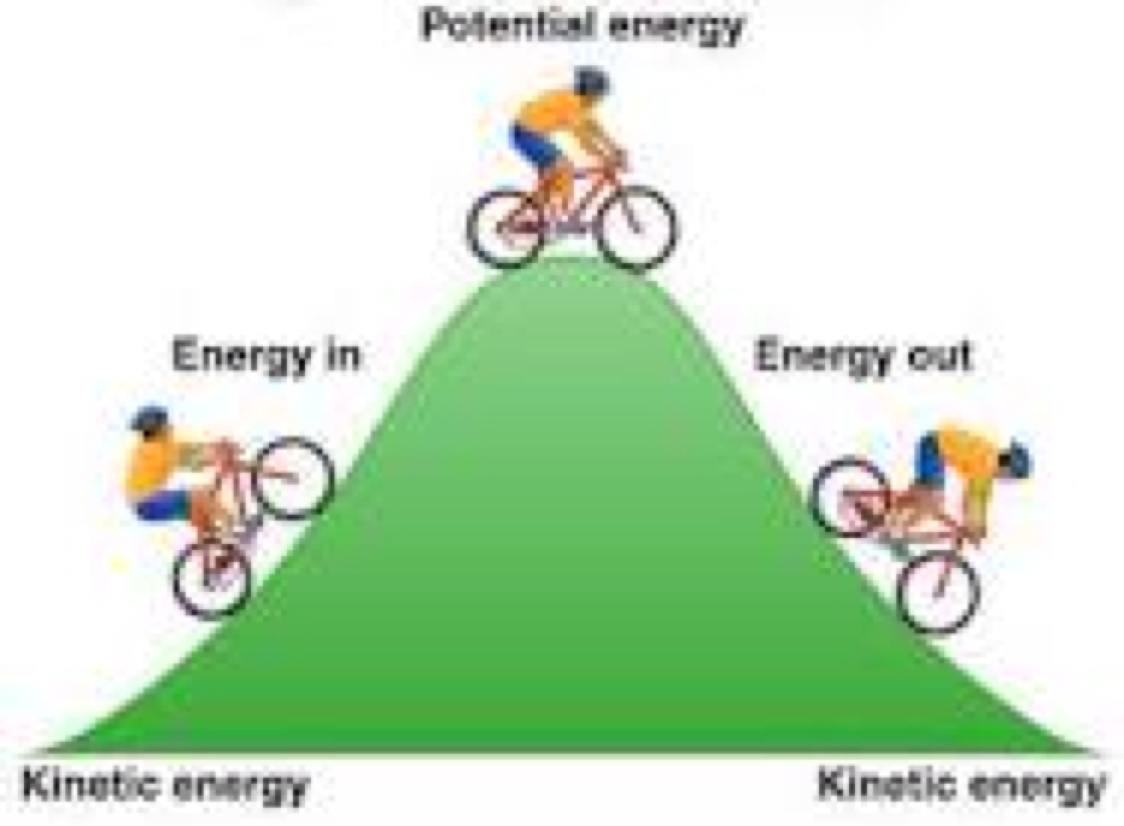
Potential And Kinetic Energy By Nate Hayden
:max_bytes(150000):strip_icc()/85375779-56a12f273df78cf7726837cc.jpg)
What Is Kinetic Energy

Kinetic Energy Https scienceterms physics kinetic energy

Kinetic Energy Https scienceterms physics kinetic energy
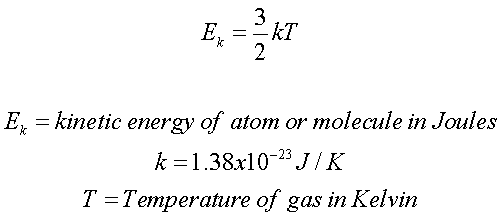
Kinetic Energy Formula Fasrfa

Which Of These Is A Measure Of The Average Kinetic Energy Of The

Average Kinetic Energy Of A Gas Molecule Is
What Is The Meaning Of Average Kinetic Energy - The average kinetic energy K is equal to one half of the mass m of each gas molecule times the RMS speed v rms squared So what is the RMS speed of the
